Neural Speech Inc.
Business Plan
Executive summary
Neural Speech Inc (NSI) develops and commercializes products that restore and enhance brain function. NSI is presently focused on restoring speech to locked-in subjects (paralyzed and mute) who are awake, intelligent and alert. Subsequent development will include a chipset in the brain to enhance cognition in normal people. NSI is a Georgia C corporation headquartered in Duluth, Georgia, USA founded in 2024.
Philip Kennedy, MD, PhD, is the founder and driving force behind NSI. He was the first to implant the human brain for long-term communication using computers in 1996, for which he received the prestigious Discover Award in 1999 and many other awards since then. He has numerous peer-reviewed scientific papers to his credit as outlined on this web site: www.neuralsignals.com . He has been featured in numerous TV and press stories, most recently in magazines such as Wired, MIT Review, Discover, Esquire and American Mind. A documentary, “Father of the Cyborgs”, produced and directed by David Burke, had its highly acclaimed USA premier at Tibeca Film festival summer 2021. Dr. Kennedy’s book, “Unlocking Erik: a freedom journey to restore speech to those suffering from the locked-in syndrome” on his decade long work with subject Erik has been published in 2021 by Nova Science Publishers (see the web site for more details). Dr Kennedy had himself implanted in July 2014 with significant data that allowed him to discover how neural signals from locked-in subjects can be decoded to produce speech.
The Team:
Jamie Hetke PhD of NeuroNexus Inc. of Ann Arbor, Michigan is developing the multi-channel version of the Neurotrophic Electrode. NeuroNexus Inc. is an essential supplier. Dr. Kennedy has been testing this multi-channel version of the electrode in rats in the Duluth, Georgia lab and the testing is now complete. He has performed the implantation with neurosurgeons Dr. Joel Cervantes and Dr. Princewill Ehirim who are both essential partners. Dr. Joel Cervantes, neurosurgeon in Belize, implanted Dr. Kennedy with four neurotrophic electrodes as discussed below.
Dinal Andreasen MSEE, an engineer from the Georgia Institute of Technology performs the recording and data analysis with Dr. Kennedy. Prof. Madeleine Lowery with student Prarthana Sakia are analyzing the data structure and function in the Bioengineering lab at the National University of Ireland in Dublin, Ireland. Daniel O’Hare with engineer Ben Griffen are building the implantable amplifiers for human implantations in Tyndall Microelectronics Center at the National University of Ireland in Cork, Ireland. Also in Tyndall is Ray Burke who will be building the photonic connections to the subcutaneous chest piece that will provide power and also decode the neural signals to produce the speech. Ryann Williams and Jeff Duke are software programmers.
The technology
The key product developed by NSI to restore communication between the brain and the outside world is known as the Neural Operating System (NOS). The Speech Prosthetic is the first product that will incorporate the NOS. The NOS could also provide the key system for restoring movement to paralyzed limbs, moving robotic arms and so on, controlling neural signals from the human cerebral cortex. However, the company will focus on cognitive enhancement as its next product. Imagine: the tail wags the dog: In other words, the dog will develop the product to help the handicapped folks (tail) and then use that (tail) development to wag the dog.
The NOS comprises a unique, proprietary Neurotrophic Electrode. It is radically different from tine (metal) electrodes (Utah array, Neuralink electrodes etc.) because it grows myelinated axons into the interior of the hollow conical electrode tip. Trophic factors are placed to encourage this growth. The electrode is implanted 5 mm below the brain surface. After three months of in-growth the signals stabilize. In one participant (ER), the signals persist for a decade until he was too ill to continue. ER died 13 years after implantation and his histology showed no sign of scarring. Instead it showed abundant myelinated axons and normal neuropil. This is unique among electrodes!
Six participants have been implanted to date beginning in 1996. The first four were implanted for communication. In 2004 locked-in participant ER was implanted for restoration of his speech, followed by Dr. Kennedy in 2014. The data from both participants confirmed that silent speech can be decoded just as easily as audible speech and statistically different from a control period of no speech. This is a key finding. We intend to implant more participants beginning in 2024.
Intellectual property protection
In the USA, the NOS system and its various components and applications are patent protected for many years and application has been made for further patents (as listed below in Dr. Kennedy’s resume). Worldwide patents are pending and will cover the EU, Canada, Japan, India, Australia and other jurisdictions. Growth factors that encourage the myelinated axons to grow into the tip of the electrode are proprietary.
History of Neural Speech Inc.
Neural Speech Inc. was founded in May 2024. The name has been secured. The three most important patents (IPs) are in Dr. Kennedy’s name only. All IPs will be signed over to Neural Speech Inc.
Competition
Regarding the electrode functionality: Other electrodes are all tine types, whereby small tines or pins are inserted into the cortex for recording from neurons. As discussed in detail in the main body of the plan, published data indicate that they will lose 85% their signals after three years (Sponheim et al 2021 [ref 6], Downey et al 2018 [ref 7]). This is due to scarring. ECoG electrodes placed on the surface of the cortex will also suffer scarring and lose signals within 2 years (Dagenhart et al 2016 [ref 7a]). Even the recent development of an electrode by Elon Musk’s Neuralink team is metal and will suffer the same fate by losing signals over time. In fact, a statement from the company indicated loss of some signals after a few months of implantation. Anecdotal accounts indicate these electrodes record for many years but the single units drop out so that lower resolution multi-units and slow frequency signals are utilized. These signals are less efficacious than the single units that our electrode records in a stable condition and for longer than any other electrode.
Cosmesis
Subjects will not shun devices that are imperceptible to them, that is, implanted under the skin or scalp. Consider how cardiac pacemakers were first rejected because they were bulky and outside the body with wires going through the chest wall into the heart. Now they are totally implanted and routinely prescribed to patients who appreciate the cosmesis. They are a big business!! Growing at 7.2% per year, the market is estimated to be over 10 billion dollars by 2025 (https://www.grandviewresearch.com/industry-analysis/pacemaker-market). Imperceptibility and cosmesis are important.
Competitive advantages
NSI’s NOS has several competitive advantages:
-
- NSI’s Neurotrophic electrode provides a stable signal, more stable than any other implanted electrode, with recordings up to 10 years when the subject could not work anymore due to his illness (see Validation paper #19 in references).
-
- It is the longest-lasting implantable electrode available (Validation paper: Kennedy et al 2017 [ref 3]).
-
- The histological analysis at 13 years when subject ER died indicates no scarring and multiple myelinated axons (Histology paper: Gearin and Kennedy 2020 [ref 2]).
-
- It is the only device that is expected to restore near-conversational speech over the long term (Validation paper: Kennedy et al 2017 [ref 3]).
-
- It provides fast computer access, enabling a subject to carry out near-conversational rate speech, undertake routine tasks and earn a living.
-
- The external hardware can be mounted on a wheelchair, making the subject mobile and sociable.
- The Speech Prosthetic is patent protected.
Product development required
First, a brief history regarding the implantable electronics: From the earliest days in 1986, we have always used implanted single channel wireless recording devices. Now, with the use of multi-channel electrodes, there is a need for multi-channel recording and hence multi-channel recording and telemetry systems. This is a major effort that has been initiated in Ireland at Tyndall Microcenter at University College Cork and at the Bioengineering Department at University College Dublin as mentioned above.
Jamie Hetke of NeuroNexus Inc. of Ann Arbor, Michigan has developed the multi-channel version of the Neurotrophic Electrode. Dr. Kennedy and engineer Dinal Andreasen have completed testing of this multi-channel version of the electrode in the lab in Duluth, Georgia.
Because the rat testing is complete, we are about to apply to the FDA for an IDE for patient implantation. Our first IDE that involved five patients has been completed and is being closed out when certain issues are sorted out. For example, the FDA wants to know what happened to the electrodes in the previous participants who are all deceased. Their concern is that the electrodes may have caused them to die (which is not so). The decision from the FDA will be available to you. Submission of a new FDA permit will commence as soon as the first FDA is finalized. In the meantime, Dr. Cervantes has paved the way for NSI to implant participants in Belize. Belize is where my implants took place.
Pricing
Blackrock Microsystems is the leading vendor of FDA approved devices including the Utah electrode array, and wired connections as well as an external wireless system, along with the appropriate computer hardware and software. A total system cost point (including Utah array electrode, wireless external system and computer and software) is US$180,000 (according to a personal enquiry made to the company). As shown below in the marketing section, we place our cost point for the total NOS system at $140,000 that is considerably less than the $180,000 that Blackrock Microsystems charges. Overall, for longevity, our system is paramount: Blackrock’s system is not a competitor because their tine type electrodes will not survive the lifetime of a subject.
For the implantable speech prosthetic:
Early adopters of the fully implantable system will be participants who are expected to pay $140,000. We will continue this as long as the market bears this cost. For insurance purposes, the benefits of the NOS include savings in life-time care and lost income. We have set up a not-for-profit foundation, Silent Speech Foundation, to raise funds prior to insurance acceptability.
The rationale underlying the insurance company’s decision is as follows: If a procedure will save more than 10 times the cost of the procedure, Medicare guidelines indicate that Medicare will pay. Medicare estimates that half the lifetime cost of maintaining a locked-in subject is $1,500,000 and lost income is about the same, $1,500,000. This latter amount is saved by the procedure since the subject who can now speak, can control a computer and talk on a phone, operate a business on the Internet and therefore will be gainfully employed. The cost benefit of an implant will be 10.7:1 (1,500,000 / 140,000) since we will charge $140,000 for the total procedure and training. A cost benefit of 10:1 or better will be recognized by Medicare for funding. Other medical insurance companies follow Medicare.
An initial configuration is to implant the Neurotrophic Electrodes alone with no wireless electronics. Instead, recording amplifiers will connect directly to the electrode. Ultimately, the system will be totally implantable, that is, when the systems have been built and tested in Tyndall Microelectronics Center, Cork, Ireland.
Market
The NOS is essentially the electrode and the implantable electronics, surgical implantation along with a computer, specialized software and training. As discussed above, it has various uses in humans. Our first target market is the speech prosthesis whereby speech will be restored to those who are locked-in (paralyzed and mute, but alert and cognitively intact).
Thereafter the uses of the NOS in disabled humans can be expanded to provide cortical control of motor prostheses (for the control of paralyzed limbs) and robotic prostheses (for controlling robotic limbs or robots). This is a possible avenue of research and we may cooperate with partners to pursue this pathway. But our main focus is on speech and, after that, cognitive enhancement.
Cognitive enhancement. Once silent speech is available, then we will to access the brain for cognitive enhancement. This is one major reason we are focused on silent speech because accessing the cloud should be private. This technology will include (a) wireless brain connections to the cloud (BrainCom) and (b) memory enhancement (using the cloud to expand the memory capacity of the human brain). It is expected that it will take several years for the initial use of the NOS as a speech prosthesis until it is used to enhance human memory. The first step in these developments is to adapt the present technology to produce a cell phone under the scalp with electrodes inserted into the speech motor cortex. A diagram of this ‘brain phone’ is shown here:
Brain phone, in brief: Neurotrophic Electrodes have been proven to persist for over a decade. About 200 neural signals are sufficient for decoding speech. A cell phone transmitter will allow the user to speak to another person or access the cloud, artificial intelligence and so on.
The potential market for the NOS is impressive. As a speech prosthetic alone, 1% market penetration will generate revenues of $18 billion.
| Prevalence | USA | Europe | Worldwide (x20 USA) | ||
| Brain Stem Stroke – locked-in | 630,000 | 870,000 | 12,600,000 | ||
| ALS – locked-in | 30,000 | 35,000 | 600,000 | ||
| Total prevalence | 660,000 | 905,000 | 13,200,000 |
At 1% market penetration: 13,200,000 x $140,000 = 1,848,000,000,000 x 0.01 =
$18,480,000,000
Overall BCI Market:
Millions of dollars are being spent on various iterations of Brain to Computer interfacing. This is a very encouraging sign of the potential of the BCI market. Examples of funding from Neurotech Reports (461 Second Street, SF, CA 94107) follow: Neuralink Inc. received $280 m as soon as the FDA gave permission for human implantation, with a valuation of $5 billion, receiving a total of $700 million. Sychron Inc received a second financing of $75 m. Cognito Inc. received $73 m. Precision Neuroscience Inc. received $41 m. Pandromic Inc. $33 m. The numbers speak for themselves.
Strategy
Since 1996, six (five plus one (myself outside FDA purvey)) human subjects have been implanted successfully with a single channel amplifier and transmitter attached to the electrodes. Subject 5 had electrodes and electronics implanted in 2004. He had the electronics replaced in Belize in 2011. He underwent further studies at NSI’s headquarters in Atlanta but he developed pneumonia, had to be put on a ventilator, and could not be studied further since his blood pressure collapsed when his head was elevated. He died in 2017.
On June 20th 2014, a sixth subject, Dr Kennedy, went to Belize and was implanted by Dr. Cervantes. Extensive data collection was completed by mid-January 2015 and the electrodes (except for the tips) and electronics were then removed. The data analysis is discussed within this business plan and confirms that the speech prosthesis is feasible with decoding of silent speech as well as audible speech. Several papers and abstracts have been published from these studies (ref 15).
The strategy is to implant a subject at no cost to the subject using the external electrode connection. The expectation is that this subject will demonstrate that speech can be produced on-line, in real time. Modifications and additions to the system will be finalized including developing the implantable electronics. Importantly, we intend to develop training software so that subjects can be trained at home. With remote access to the subjects’ computer, training can be coached from NSI headquarters no matter where in the world they live. This will reduce costs and broaden our subject base.
Since we will go to Belize to implant, the initial lack of FDA approval will not delay commercialization. Subsequent FDA and European permits, plus permits from other countries, will broaden our net of subjects.
Brain Implant Centers
Until recently, all implants were carried out at hospitals near NSI’s headquarters in Atlanta, Georgia. NSI intends to establish worldwide Brain Implant Centers (BICs) where the Speech (and other) prostheses can be implanted. Subjects will be trained remotely from the lab in Duluth, Georgia. Dr. Kennedy’s implant operation was performed successfully at the Brain Implant Center in Belize City, Belize.
NSI intends to set up a worldwide network of implant centers. Locations and surgeons have already been identified in Oxford (UK) and Belize City (Belize). The BICs will be independent entities, and they will buy the NOS from NSI and also pay NSI a license fee. The BICs have no set up costs: A simple approval from the surgeon is sufficient.
Production
The electronic components of the NOS will be manufactured by the Tyndall group in Ireland. Other components may be manufactured elsewhere such as NeuroNexus that will manufacture the electrodes. The external computers will be provided by Neuronexus Inc. or Neuralynx Inc. The training will be done remotely by personnel at Neural Speech headquarters.
FDA approval
NSI has an FDA IDE (G960032S). This IDE is in suspension as discussed above. We will apply for EU and other country approvals to allow us to implant subjects within Europe and other countries. In the meantime, and to avoid delay in commercialization, subjects will be implanted in Belize where permission was obtained for my implantation and has presently been renewed by Dr. Cervantes. Implanted subjects will be trained in their homes with instructions on line from NSI headquarters in Atlanta Georgia. Much of the training will be automated.
Management
NSI is being run by the founder Dr. Phil Kennedy. To assist the implantation and training a neuroscientist will be hired. Administration will be provided by a staff administrator. As the project develops, managerial and technical staff required to run the company will be hired.
Funding required
NSI has benefited from $4.4 million in research funds from the National Institutes of Health (NIH) in the USA to develop the NOS. At present, research is being funded from Dr Kennedy’s personal funds to the tune of $450,000.00.
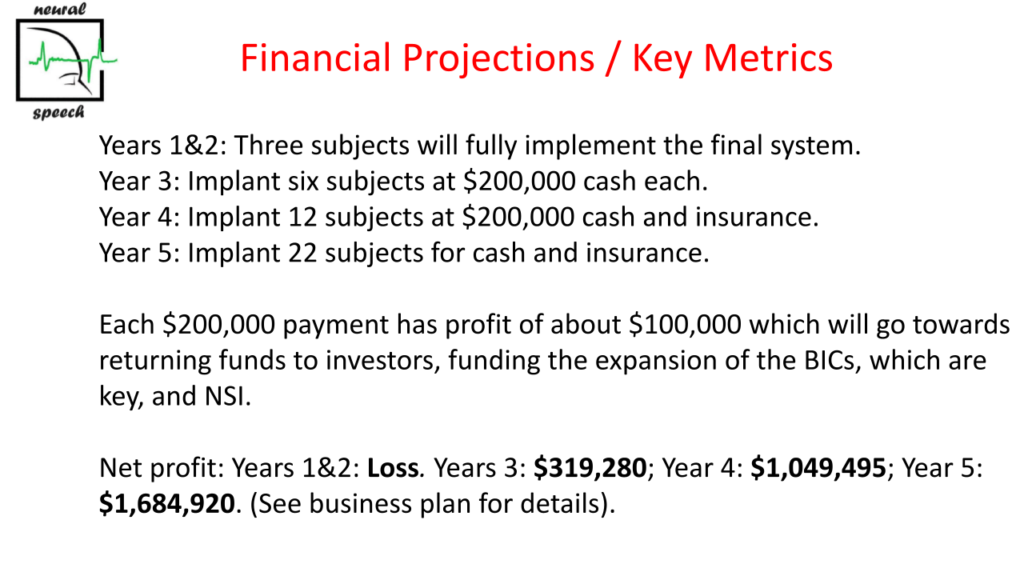
The company is now seeking to raise $1,000,000.00 for three patient implants in Belize with an option on 10% of the shares of Neural Speech Inc. Post implants, round 2 funding will be sought. Pre-funding valuation is $4.5 million. The company is offering a significant percentage of the ordinary shares of Neural Speech Inc. yet to be determined with a valuation of $10,000,000. (It should be noted that Elon Musk’s Neuralink was funded for $280 million and is now valued at $5 billion after a recent round of funding.) These funds will be used to implant more locked-in subjects to produce speech in 2025, finalize FDA permission, set up the remote computer system, develop training software using machine learning, develop the implantable amplifier and decoding electronics. A more detailed budget outline is available.
Risk
The funding of NSI’s development is a low-risk project because:
-
- It is the only device available to restore near-conversational speech over a lifetime
-
- enabling richer and more human relationships with family and friends
- enabling subject to interact more effectively in a work environment
-
- It is the only device available to restore near-conversational speech over a lifetime
-
- System to receive the decoded speech (a cell phone is likely) will be mounted onto a wheelchair to enable mobility and sociability.
-
- Provides computer access via speech
-
- enabling everyday tasks
- enabling subject to work on the Internet and become employed
-
- Provides computer access via speech
-
- Electrode is anchored in place because the brain tissue grows through it and anchors it in place. And there is no scarring inside the electrode tip, so it
-
- is the most long lasting on the market and
- provides a consistently stable signal better than any other electrode.
-
- Electrode is anchored in place because the brain tissue grows through it and anchors it in place. And there is no scarring inside the electrode tip, so it
- Six patients have been implanted with the original electrode with no significant complications and no electrode failures.
Contact Details
Philip Kennedy, MD, PhD
CEO, Neural Speech Inc.,
3400 McClure Bridge Road, Suite D402,
Duluth, Georgia 30096, USA
Phone: (+1) 404-771-2034
Email:Phlkennedy@aol.com
Web site: www.neuralsignals.com
Table of Contents
1.3 Experience-led Development 7
2 The Neural Operating System 9
2.1 History of Implantations 9
2.2 Intellectual Property Protection 11
2.5 Product Development Required 13
5.4 Active Research Consultants 26
5.5 Investigational Review Board 26
5.6 Data Safety and Monitoring Committee 27
6 Funding & Financial Projections 29
8 Appendices …………………………………………………………………………… 34
- The Company
Neural Speech Inc (NSI) develops and commercializes products that restore and enhance brain function. NSI is initially focused on restoring speech to locked-in subjects who are awake, intelligent and alert, but paralyzed and mute.
History of Neural Speech Inc.
In October 2022 Dr. Kennedy’s company, Neural Signals Inc., was funded by Dennis O’Leary with an LLC mechanism called Neural Logistics LLC (NL). NL had rights to 50% of Neural Signals Inc and 50% rights to Dark Pulse Inc., Dennis O’Leary’s public company. Mr. O’Leary was contracted to fund $350,000 but only funded $250,000 leaving Dr. Kennedy to supply $450,000 from his personal funds to keep the research afloat from April 2023 to now. This was an untenable situation. So, Dr. Kennedy, rather than spend $5,000 to $40,000 in arbitration, and thousands more in court, simply dissolved Neural Signals Inc. in April 2024. This dissolved Neural Logistics LLC by default. So Neural Speech is about to be founded in May 2024. The name has been secured. The three most important patents (IPs) are in Dr. Kennedy’s name only. The IPs will be signed over to Neural Speech Inc.
-
The NSI Mission
We normally receive input via our eyes, our ears, our senses of touch, pain, vibration and proprioception (position of a limb). Our output is through speech and movements that utilize skills such as speaking, writing, walking, running and touching others. All these activities are examples of two way communications between the brain and the external world by using our body parts.
For most of us, this brain-world communication is never a problem. However, the link between the brain and the world can be damaged or broken by an accident (eg, spinal cord injury) or other event (such as a brainstem stroke), or by a disease such as ALS.
As a result, a subject can become locked-in, that is, unable to communicate with the outside world to a greater or lesser extent even though his or her brain is still functioning normally in all other respects.
The mission of Neural Signals Inc (NSI) is to restore and enhance this essential link between the brain and the outside world, specifically to restore speech.
NSI has done so by developing a speech prosthetic. NSI intends to manufacture and sell the prosthetic, implant them in subjects and deliver the training needed to enable subjects to use them effectively.
After NSI has developed the speech prosthetic, it may proceed to use the NOS for restoring movement, controlling paralyzed limbs, robotic arms, exoskeletons, and so on for patients. In parallel it will develop products that enhance normal brain functions such as memory, calculation, wireless brain communication (cell phone) among other applications.
As of now, NSI has successfully developed a speech prosthetic that requires funding to prove its functionality in locked-in subjects.
-
Corporate Values
Neural Signal’s corporate values are based on (a) adherence to the highest medical ethics, (b) the use of cooperative research in technical development, and (c) ensuring that all products and techniques developed by the company deliver long-term benefits for human beings. Our mantra for the speech prosthetic is “unmute the mute”. As physicians and engineers, we believe in the Hippocratic oath and a similar Technocratic oath. The main thrust of the oath is, first, do no harm.
-
Experience-led Development
Dr Philip R Kennedy is the founder of Neural Signals Inc and the company’s chief scientist officer (CSO). He is driven by a long-time passion to help paralyzed and mute people improve personal communication by speech restoration.
He was the first scientist, along with the late neurosurgeon Dr. Roy Bakay, to implant an electrode into a human brain. He did so in 1996 in order to provide a subject with long-term communication using computers. For this singular achievement he received the Discover Award in 1999. His aim now is the restoration of human speech.
Dr Philip Kennedy leads the field in invasive brain computer interfacing. He has published numerous peer-reviewed scientific papers (see appendix 6) and has been featured in numerous press and TV stories over the last few years, including magazine articles in recent issues of Discover, Esquire and American Mind. A documentary released in 2020 called “The Father of the Cyborgs”, features him and his achievements. He has also written a book describing his research with Eric and describing his own brain implantation called “Unlocking Erik: A freedom journey to restore speech to those with the locked-in syndrome”. It is published by Nova Science publishers. A link to the book is found on our web site, www.neuralsignals.com.
The development of NSI’s products and surgical techniques is driven by Dr Philip Kennedy’s long-time in-depth experience in these fields. After 38 years of intensive research and development, NSI is a repository of knowledge and expertise on the repair and enhancement of communications between the brain and the outside world.
-
The Neural Operating System
The product developed by NSI to restore communication between the brain and the outside world is known as the Neural Operating System (NOS). It consists of electrodes implanted in the brain with associated implanted electronics and external hardware and software with a wireless link between the implanted electronics and the external equipment.
Neural Signals’ NOS comprises a unique, proprietary Neurotrophic Electrode, a unique surgical implantation technique and unique hardware, software and speech restoration training programs.
Implanting the Neurotrophic electrode requires special surgical techniques which have been developed by Dr Kennedy. Once the electrode is implanted into the subject’s brain it takes three months for the brain tissue to grow into and through the electrode tip and anchor the electrode, after which recording of signals and training can begin. This training is essential to enable the subject to use the system to communicate with a computer so as to produce phrases through the computer speakers.
The earliest applications of the NOS focused on enabling a locked-in subject to control the cursor of a computer and hence spell out simple words. The current focus is on the restoration of speech.
Indeed the neural operating system has been developed by Neural Speech as a platform for multiple applications, as it provides for the extraction of multiple signals directly from the brain in real-time. It will eventually enable direct brain control for multiple neural prosthetic applications such as controlling computers and other devices, regaining speech, and controlling the movement of paralyzed limbs, artificial limbs or robots.
-
History of Implantations
In 1986 Dr Phil Kennedy began experimenting with the implantation of rats in the Georgia Institute of Technology, Atlanta, followed by more years implanting and training monkeys at the Yerkes Primate Center in Atlanta. A total of 42 rats and 8 monkeys were implanted between 1986 and 1992. These implantations showed that the signals from the electrode could endure for 16 months, ie until the monkeys damaged the implants. Now we have a verified decade long implantation in a human (ref 19, 21-23).
NSI was established in 1987 and was awarded its first patent in 1989. Human implants began in 1996. Since that time five locked-in subjects have been implanted, plus one normal subject. All operations were successful.
The first, a 52-year old female with ALS, was implanted with the earliest version of the Neurotrophic Electrode in the part of her brain which once controlled her now paralyzed left arm. Before she died from her underlying disease, she showed that neural signals could be turned on and off under her own control.
The second subject, a 54-year old male paralyzed due to a brainstem stroke, was implanted in 1998. He was able to communicate through a computer by first imagining he was moving a mouse with his left hand, and then just by thinking about moving the cursor -the first ever demonstration of human cortex plasticity. He could control the mouse cursor in two dimensions and spelled out words on the computer. He was the world’s first cyborg because he was the first to control a computer without using limbs or speech.
Two further male subjects aged 43 and 45 were implanted in 2000 and 2001 respectively. One showed that his neural signals were related to residual muscle activity in his contralateral arm. The other, despite a successful operation, showed that no signals appeared since he suffered from mitochondrial myopathy which, unknown to the field, also affected his brain and not just his muscles.
Then, in 2004, the fifth subject, a 22-year old mute and fully-paralyzed male, had the NOS implanted in that part of his cerebral cortex where speech articulators are controlled.
The recorded neural signals were transmitted to a computer, decoded into human speech and communicated through the computer speakers, thus proving the viability of the NOS. The diagram at the bottom of this page illustrates the process.
This subject could listen to the sound of a phoneme … phonemes are the building blocks of language … and then say it in his head. He was able to produce neural signal patterns that allowed identification of over half the English phonemes. He was also able to move a cursor across the computer screen from one phoneme sound to the next with 80% accuracy.
This subject has since died due to his underlying stroke. The signals were still present and functional for 10 years after implantation (December 22, 2004), which proves that the electrode provides (a) stable signals for long-term recording and (b) that the recorded signals can be used to decode speech. In addition, the histology of the electrode implant demonstrated no scarring, but copious amounts of myelinated axons that had grown into the center of the electrode tip (Ref #23).
We learned that many more signals are needed to restore near-conversational speech ….. that is, implantable multichannel electronics are essential for continued development.
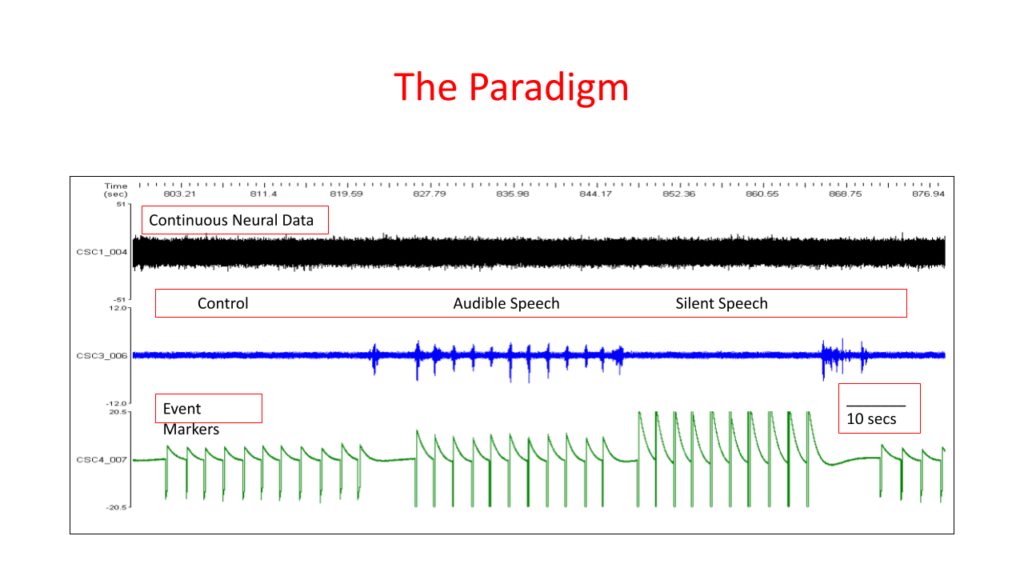
Dr. Kennedy’s speech cortex was implanted in 2014 as illustrated below. Results from these studies indicate that phonemes, words and phrases can be detected when spoken silently (in his head) just as they can when spoken audibly (refs 19,21). In addition, when compared to a control period of no speech, there is a statistically significant difference between the control period and both the audible and silent speech, but no statistically significant difference between audible and silent speech.
Dr Kennedy has presented these results over the years to meetings of the Society for Neuroscience and at other scientific meetings and has published the results (Refs 19-21-23).
-
Intellectual Property Protection
The technology used in the neural operating system is protected with proprietary knowledge and several patents.
NSI’s has intellectual property protection on the NOS as used for the speech prosthetic which is valid until 2027 under US patent number #7,275,035 B2 issued in 2007. This patent protects the prosthetic used in speech restoration. The neurotrophic factors inserted into the
electrode tip are proprietary. A recent patent covers the chipset in the brain that enhances cognition by allowing the person to access the internet, ChatGPT, and so on, merely by speaking silently. That is why assisting locked-in persons to speak will be parlayed into persons who are not locked-in.
A list of 11 patents is below. Worldwide patents are pending, in jurisdictions such as the EU, Canada, Japan, India, Australia and so on.
-
Competition
Locked-in persons are subjects who are both paralyzed and mute. This means that they cannot move their limbs and cannot speak. But they can see, hear and think normally.
There are no devices on the market that can provide a completely locked-in subject with near-natural speech ability. However, they can be helped by both non-invasive and invasive communication systems.
There are a number of companies which offer solutions that provide a completely locked-In subject with computer access and therefore basic, but very slow, communication. These assistive and augmentative communication products are non-invasive, ie., they are not implanted inside the body.
One of these companies, Brain Fingers, recently launched a non-invasive device called MUSE. This is a headband that detects electrical signals from facial muscles, eye movements, and brainwaves (EEG). The software decodes these and allows the subject to control a computer, hands free.
Another similar technology is being developed by Niels Birbaumer. His technology is called a Thought Translation Device (TTD). It is a cap which brings the electrodes into contact with the subject’s scalp. It picks up EEG signals from the subject’s brain and uses these to control a computer.
There is a computer device that uses eye movements to control the computer – Tobii Inc from Sweden. This is an advanced computer system but it does not restore speech.
All these devices require a training period during which the subjects learn how to direct their thoughts so that they are able to control the devices. Once this is established, they can use it for computer access and basic communication. In both case, communication is extremely slow.
Jon Wolpaw and his group at the Wadsworth Center in Albany, New York, have developed similar devices.
Other groups are developing brain surface electrodes (ECOG) that provide a signal that has higher resolution than EEG signals. These, however, are much less precise than the single units NOS uses to record from the brain. A review of all these systems is provided in the reference list (#22).
Invasive systems are systems that are implanted inside the subject’s brain.
One group led by Eric Aartnouse (Utrecht, Netherlands) uses electrodes placed on the surface of the brain. Another group led by Ed Chang (San Francisco) does the same thing. These are called ECoG (ElectroCorticographic) devices and results in scarring over months and years (ref 17). The Neurotrophic electrode on the other hand has been shown to provide signals for a decade! There is no scarring.
NSI’s NOS is an example of an invasive system, as are the ECOG systems. Cyberkinetics Inc is working on invasive products to provide control of movement of paralyzed limbs, speech and robotic arms. Cyberkinetics uses the Utah Array (now known as the Blackrock Array) which is an electrode that is quite different from the NOS electrode in that it uses metal tines inserted into the cortex. Tine type electrodes generate scarring and micro-movements and so neural signals are gradually lost over months and years. Cyberkinetics has recently ceased its activities but the research is being undertaken by Braingate Inc.
This loss of signal has occurred in all Cyberkinetics’ monkey and human implants with 85% loss by the end of year 3, though there can be multi-units (ie. two or more signals recorded together) with some function at 5 years (See Appendix 3 for abstract) and ref 6 & 7. As Cyberkinetics has stated: signal quality was considerably lower than that seen in the months just after array placement.
More recently, Neuralink has devised an electrode can record over a thousand single units in monkeys (ref 9). However, the first patient implant in 2024 resulted in loss of some signals. However, adjustments were made and the recordings are continuing.
Three or five years is not the lifetime of the subject. Our longest lasting subject was 10 years until he died in 2017 of his underlying disease. Indeed, signal loss is clearly not acceptable for a device that needs to endure for the lifetime of a subject, which, in the case of a 20-year old quadriplegic, would be at 50 years or more. Thus, there is no satisfactory alternative to the Neural Signals’ NOS.
-
Competitive Advantages
The current non-invasive technologies for completely locked-In subjects offer computer access to provide slow and very basic communication. However, most do not offer speech.
Although basic communication by moving a computer mouse through a range of words, letters or symbols is useful to the subject, it is limiting in terms of forming and maintaining human relationships and in allowing the subject to participate in the world in real-time because it is inherently slow.
NSI’s electrode is more effective than other electrodes currently being used due to three key factors: (1) the first is that it is anchored by brain tissue which grows into and through the hollow electrode tip which means it is held securely in place so that recordings are not lost;
(2) the second factor is that no scarring has been found on histological examination using light or electron microscopic examination in rats and monkeys (ref 1). This is also true for the fifth subject who was implanted for 13 years (with 10 years of recording) (ref 2);
(3) the third factor is that the wire lead from the electronics to the electrode tip is coiled to reduce strain, thus ensuring longevity.
These factors result in robust, long-lasting signals from our electrode. NSI has the longest recorded activity from an electrode.
The benefits of the NOS may be summarized as follows:
-
- It is the only device available to restore near-conversational speech over a lifetime
-
- enabling richer and more human relationships with family and friends
- enabling subject to interact more effectively in a work environment
-
- It is the only device available to restore near-conversational speech over a lifetime
-
- System can be mounted into a wheelchair to enable mobility
- Electrode is anchored in place with brain tissue and has no scarring inside the electrode tip, so it
-
- is the most long lasting on the market and
- provides a consistent signal better than other electrodes.
-
-
Product Development Required
Neural Signal Inc (NSI) sought from the very beginning in 1986 to produce a long-term electrode that could be used over the subject’s lifetime. Its success is due to the fact that the brain grows into the electrode tip and is held there. Data has been obtained and published showing stability of neural activity over the long term. (ref 3).
NSI’s success to date in restoring speech gives us confidence that we will restore useful speech at a near-conversational rate. By useful speech we mean the ability to use 100 or more common English words or phrases at a near conversational rate that can be understood by listeners.
As a reminder, Dr. Kennedy had his speech area implanted in 2014. Results from these studies indicate that phonemes, words and phrases can be detected when spoken silently (in his head) just as they can when spoken audibly (ref 15). In addition, when compared to a control period of no speech, there is a statistically significant difference between the control period and both the audible and silent speech, but no statistically significant difference between audible and silent speech.
The limiting technical factor in the development of the NOS is the lack of a multichannel electronics package and thus a severely restricted number of channels. The funds raised will be used to develop a multi-channel recording system.
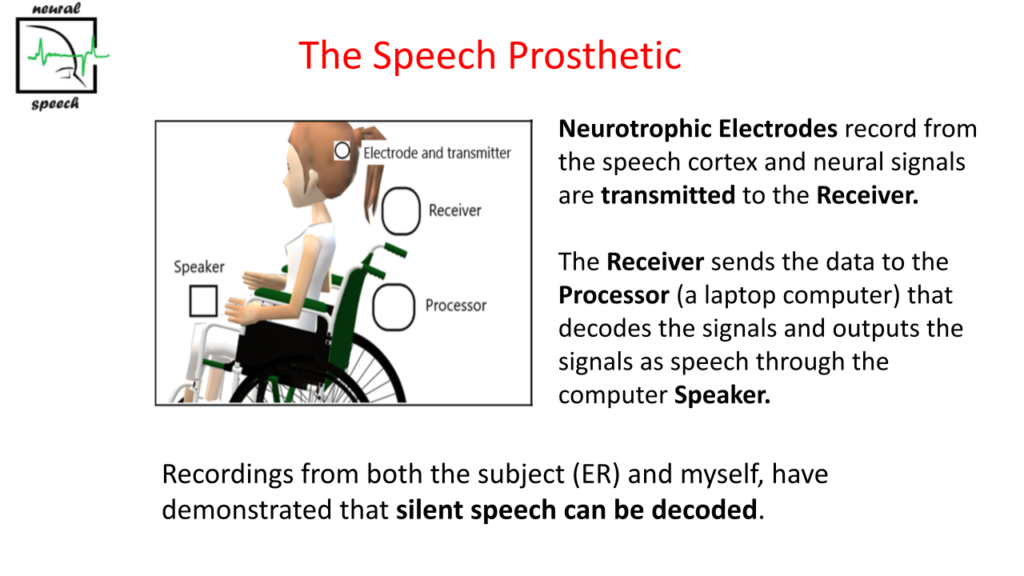
We intend to develop the NOS so that the whole unit is mounted on a wheelchair and the subject can take it with him or her to the outside world as shown here.
The ‘Receiver’ receives the neural signals from the ‘Electrodes and transmitter’. It transfers the data to the ‘Processor’. The ‘Processor’ is essentially a laptop or phone that decodes the neural signals, detects the speech and outputs the speech through the laptop ‘Speaker’.
The map of activity:
1: Increase the number of recording channels from 2 or 4 to 16 per electrode (with NeuroNexus Inc).
2: Implant these electrodes in rats and record the neural signals (completed).
3: Implant three locked-in persons in Belize with Dr. Cervantes.
4: In parallel, develop the implantable multichannel electronics.
5: Close out our FDA IDE (G390063S).
- Apply for a new IDE from the FDA.
-
Future Products
NSI is currently focusing on using the neural operating system (NOS) to restore speech. Once this is achieved, the technology will be developed further into areas such as control paralyzed limbs, control robots, control robotic limbs as applied to humans, and then to future applications such as memory enhancement and wireless brain connections (phone) including Internet directly to and from the brain.
The table summarizes the expected development path of future products.
| Use of NOS and/or Neurotrophic electrode | Prevalence
(## of subjects) |
Revenues per subject | Projected revenues at 1% market penetration | Income initiation |
| 1-Speech prosthesis | 9,900,000 | $140,000 | $14 billion | Year-3 |
| 2-Motor prosthesis | 18,750,000 in
Shared market |
$130,000 | $281 billion in
Shared market |
Year-7 |
| 3-Robotic prosthesis | $150,000 | Year-8 | ||
| 4-BrainCom prosthesis | 8+ billion | $10,000 | $7 trillion | Year-10 |
| 5-Memory prosthesis | 8+ billion | $10,000 | $7 trillion | Year-15 |
The next section will go into further detail on the first two markets.
-
The Markets
The NOS and/or its components will have a host of uses. Initially, however, it will be used as a speech prosthetic for locked-in subjects.
-
The Locked-in Market
There are numerous conditions and diseases which can cause a subject to become almost locked-in or completely locked-in and for whom the NOS would be a suitable solution.
These conditions and diseases include brainstem stroke, amyotrophic lateral sclerosis (ALS), high spinal cord injuries high (tetraplegia), complete and irreversible Guillian Barre Syndrome, axonal neuropathy, muscular dystrophy, cerebral palsy, dystonic cerebral palsy, specific cortical strokes, some primary progressive atrophy and brain tumors in specific areas, among others.
The three major conditions are brainstem stroke, ALS and high spinal cord injuries. Hence, in estimating the potential market for the human use of the NOS, the focus is on these conditions.
-
Stroke
A stroke is the rapidly developing loss of brain function due to a disturbance in the blood supply to the brain. This can be due to ischemia (an inadequate blood supply to an organ or part of the body) caused by a thrombosis (clot in the vessel) or an embolism (clot travelling into the vessel from, commonly, the heart) or due to a hemorrhage.
As a result, the affected area or areas of the brain are unable to function, leading to inability to move one or more limbs on one side of the body, inability to understand or formulate speech or inability to see one side of the visual field. The symptoms of a stroke are different depending on the area of the brain in which the stroke is located.
Stroke is the leading cause of adult disability in the USA and Europe. There are 795,000 strokes a year in the USA, while the number (prevalence) of surviving stroke victims in the USA is almost 5 million.
A cortical stroke is one which occurs in the upper part of the brain, the cerebrum. The cerebrum is the largest and most developmentally advanced portion of the brain. It controls a number of higher functions, including speech, emotion, the integration of sensory stimuli, initiation of the final common pathways for movement, and the fine control of movement. Thus, if the stroke occurs in the output pathway, deep in the brain, the speech motor cortex is intact and can be implanted. This is termed a brainstem stroke. It is one which involves the lower part of the brain and disconnects the cerebrum and spinal cord, essentially blocking all ability to move, even of the face sometimes.
Of the 795,000 strokes per year in the USA, about 520,000 subjects survive and 210,000 of the survivors have speech problems of varying degrees.
In about 10% of these subjects (21,000 subjects) the stroke is in the brainstem and is severe enough to put the subject into a locked-In state, in which the subject can sense stimuli, see and hear but cannot respond. The lack of independence and inability to interact with the world place an enormous amount of pressure on the subjects’ relationships, finances and quality of life.
These subjects would benefit from the NOS in multiple ways. It would allow them near-natural speech communication and computer access for both work purposes and simple daily tasks such as turning the television or lights on and off, calling for a caregiver, etc. This provides a US market with an incidence of 21,000 annually.
An estimated 1.1 million strokes occur each year in Europe, and about 860,000 of these subjects survive, with 290,000 suffering speech impairment.
If 10% of these subjects are affected significantly enough to benefit from the NOS, this provides a European market with an incidence of about 29,000 subjects per year.
According to the World Health Organization, 15 million people suffer a stroke worldwide each year, which is 20 times the number of stroke incidents in the USA. Of these, 5 million die, leaving 10 million to live with the effects of stroke, with 3,400,000 suffering from a speech impediment.
If 10% of these 10,000,000 subjects are affected severely enough to benefit from the NOS then there is a global market of 1 million subjects annually. However, to be conservative, we can simply multiply the USA data by 15 to find the estimated overall world figure, giving a smaller number of 315,000 potential subjects.
This provides us with a US market of about 21,000, a European market of 29,000, and an overall global market of 315,000 annually. The latter figure includes the US and European figures.
-
ALS
Amyotrophic Lateral Sclerosis (ALS), also known as motor neuron disease or Lou Gehrig’s disease, is a progressive neurodegenerative disease that affects nerve cells in the brain and the spinal cord. It is characterized by the gradual onset of paralysis rendering the subject unable to move or speak, while cognition remains intact in 50% of patients. The degeneration can spread to the frontal lobes producing a frontal lobe syndrome making 50% of the subjects unsuitable for implantation.
Motor neurons reach from the brain to the spinal cord and from the spinal cord to the muscles throughout the body. When the motor neurons die, the ability of the brain to initiate and control muscle movement is lost. The progressive degeneration of the motor neurons in ALS eventually leads to death if subjects chose not to accept a ventilator.
In the most typical form of ALS, the disease is first indicated by awkwardness and difficulty in tasks requiring fine finger movements, stiffness of the fingers, and slight weakness or wasting of the hand muscles or stumbling during walking. In the later stages of the disease, after about 4-5 years on average, the subject, while alert, is totally helpless – unable to speak, swallow, or move the limbs. At this point the subject needs to make the decision whether or not to accept a ventilator for breathing. Less than 10% of ALS patients take this opportunity.
If an ALS subject accepts a ventilator their life expectancy is extended indefinitely. At NSI, we believe that if ALS subjects are implanted with the NOS and the ability to interact with family and friends using near-natural speech, a much higher proportion of subjects will choose to accept the ventilator. And there will be no reason why they cannot open a business on the Internet.
ALS has a worldwide annual incidence of 0.4 to 1.8 per 100,000 population and a prevalence of between 4 and 6 per 100,000 population.
Approximately 5,600 people in the US are diagnosed with ALS each year and it is estimated that as many as 30,000 Americans have the disease at any given time. About 5,000 people die from the disease every year because they decide to not use a ventilator to stay alive.
The progressive nature of ALS means that in or around the third to fifth year of the disease the subject loses all muscular control and speech ability and use of the NOS (speech prosthetic) would be necessary to communicate. Each year, approximately 5,600 people in the USA reach the stage where they need to decide whether or not to accept the ventilator. We conducted a survey of ALS patients. Nine ALS patients who expect to be locked-in want the speech. Of those already locked-in, 2 wanted it, 1 did not. Of those not diagnosed with ALS, the majority indicated they would accept the speech prosthetic (34, versus 2 not wanting it). These are small overall numbers but the majority clearly favor some relief from their locked-in state by accepting the speech prosthetic. The survey described the surgery and post-op training in some detail. Most respondents also indicated they would raise funds to get the surgery.
It is estimated that the European incidence for ALS is 8,400 per annum, and the global incidence is 132,000 per annum.
Table-1 below summarizes the annual Incidence and prevalence of locked-in stroke and ALS subjects.
The prevalence of brainstem stroke and ALS subjects is based on a 30-year lifetime survival of brainstem strokes and ALS, with modern medical care. As can be seen from Table-1, the number of subjects needing the NOS as speech prosthesis globally is almost 10 million.
Table-1: Subjects locked-in due to brain stem stroke and ALS
| USA | Europe | Total Worldwide | |||
| Annual incidence | |||||
| Brain Stem Stroke – locked-in | 21,000 | 29,000 | 315,000 | ||
| ALS – locked-in | 5,600 | 8,400 | 84,000 | ||
| Total incidence | 26,600 | 37,400 | 447,000 | ||
| Prevalence | |||||
| Brain Stem Stroke – locked-in | 630,000 | 870,000 | 9,450,000 | ||
| ALS – locked-in | 30,000 | 35,000 | 450,000 | ||
| Total prevalence | 660,000 | 905,000 | 9,900,000 |
-
Spinal Cord Injury
There are 12,500 cases of quadriplegia (paralysis of both arms and legs) and another 12,500 of paraplegia (paralysis of the lower part of the body) per annum in the USA.
With modern medical care, the lifetime of these subjects approaches a normal lifespan of over 70 years when injured in the 3rd decade of life. Most spinal cord injuries occur in young people. The USA prevalence of spinal cord injury is 1,250,000.
According to the Spinal Cord Injury Information Network the lifetime economic impact of being paralyzed is $5,885,113 per patient. This is based on cost of care, $2,924,513, and lost income, $2,960,600. Clearly, this is an enormous economic burden as well as a human problem.
Rounding up the cost to $6 million, the cost to the economy of caring for 1,250,000 persons is $7.5 trillion over their lifespans.
A small percentage of these subjects will have severe, high level spinal cord injury and be unable to speak. Because there are no adequate incidence and prevalence data on high spinal cord injuries, they are noted, and we pass on.
-
Other Markets
Once the initial markets for the NOS as a brain prosthetic for locked-in subjects and those with severe spinal cord injuries, other markets will be entered on a commercial basis.
These markets include motor prostheses for the control of paralyzed limbs and robotic prostheses for the control of robots. Applications in the more distant future (ie, at least a decade away) will include wireless-brain connections (BrainCom or phone) and memory enhancement, ie using external hardware to expand the memory capacity of the human brain.
Table-2: Estimated overall market
| Use of NOS and/or Neurotrophic electrode | Prevalence
(as## of subjects) |
Revenues per subject | Projected revenues at 1% market penetration | Timetable |
| 1-Speech prosthesis | 9,900,000 | $140,000 | $14 billion | Year-3 |
| 2-Motor prosthesis | 18,750,000 | $150,000 | $281 billion | Year-7 |
| 3-Robot prosthesis | $150,000 | Year-8 | ||
| 4-BrainCom prosthesis | 7+ billion | $10,000 | $7 trillion | Year-10 |
| 5-Memory prosthesis | 7+ billion | $10,000 | $7 trillion | Year-15 |
Overall BCI Market:
Millions of dollars are being spent on various iterations of Brain to Computer interfacing. This is a very encouraging sign of the potential of the BCI market. Examples of funding from Neurotech Reports (461 Second Street, SF, CA 94107) follow.
Neuralink Inc. received $280 m as soon as the FDA gave permission for human implantation, with a valuation of $5 billion.
Sychron Inc received a second financing of $75 m.
Cognito Inc. received $73 m.
Precision Neuroscience Inc. received $41 m.
Pandromic Inc. $33 m.
The numbers speak for themselves.
-
Strategy
4.1
Funding Round 1:
Year 1 (2023): [A] The Neurotrophic Electrode has been modified by NeuroNexus Inc. and tested during 2023 in rats. The result is a dramatic increase in single unit recordings from 20 or 40 to an average of 400 using the same tip dimensions.
[B] The implantable electronic system has been initiated and continued over a two to three year period.
Year 2 (2024): The electrode will be implanted in three locked-in persons in Belize under the aegis of Dr. Cervantes. The Neuralynx recording system will be plugged into the electrode and the data will be decoded using machine learning decoding software. Speech output is expected from the system.
Year 3: The implantable electronics will be tested in large animals for safety and efficacy according to FDA guidelines.
4.2
Funding Round 2:
Year 4: [A] Implantation and recording for speech production will commence with the implantable electronics attached to the electrodes. It is expected that revenues will commence.
[B] Brain Implantation Centers (BICs) will be initiated in addition to the Belize BIC.
4.3
Brain Implant Centers
A central corporate strategy for worldwide distribution of the speech prosthetic involves Brain Implant Centers (BICs). The centers will have trained neurosurgeons who will implant the Speech Prosthetic. The subjects will be trained from Neural Signals headquarters in Duluth. Georgia using remote computer access no matter where in the world the subject lives. At the subject’s home, an assistant will be trained to turn on the system and advised by remote audio-visual systems, such as Zoom.
Belize Implant Center: The first BIC is in Belize. Two surgeries (one on subject 5 and other on Dr. Kennedy) have been performed there successfully. This is the Belize BIC with neurosurgeon Joel Cervantes, MD. There is no cost to set up. Permission from the local medical board and from the local neurosurgeon.
It was in the Belize center that subject 5 was operated on in order to replace his electronics. Dr Roy Bakay of Rush Medical Center in Chicago accompanied Phil Kennedy to perform the operation with Dr Cervantes. Dr Bakay performed all the monkey implants and first three human implants before leaving Atlanta for Chicago. The facilities at Belize are adequate, the care is excellent and the outcomes were successful. We look forward to performing further surgeries there and returning the subjects to their homes for standalone training with remote access assistance from NSI headquarters. Dr. Kennedy received his implants in Belize in 2014. FDA approval is not required for US citizens to undergo surgery outside the FDA’s jurisdiction. I discussed this with the FDA prior to taking subject 5 to Belize. Local permission has already been obtained for the first two subjects and will likely be extended for other subjects.
Atlanta Implant Center: With FDA approval renewal, Dr. Ehirim, neurosurgeon, will perform the surgery that he has performed in the past. Five of the subjects were implanted in the Atlanta area. Implants in Atlanta and the USA will have to await FDA approval if performed in the USA. In the meantime, US subjects will travel to Belize for implants.
Oxford University Implant Center: In England, Dr Alex Green at Oxford University’s Neurosurgery Department is interested in NOS implantation. UK government approval will be required.
Cyprus: There is a possibility of availing of surgical facilities in Cyprus.
Ireland: There is a possibility of availing of surgical facilities in Ireland.
The worldwide BICs will be based in private or university hospitals. Each one is a center where the implant is performed. The subsequent training is performed remotely from Neural Signals headquarters. Neural Signals will supply the BICs with the electrodes and electronics and will pay the BICs to perform the implants. The subject will pay Neural Signals.
BICs will be the core sales conduits for the speech prosthetic. Thus, multiple BICs are expected to be established in various countries around the world as the project progresses. Prior to setting up these BICs, travel is possible for locked-in subjects with some precautions. Locked-in subject 5 travelled to Belize without difficulty. So having just one BIC in Belize allows the implants to continue.
Principles of operation and agreements with the centers are provided in the appendix 5.
4.4
Promoting the NOS
Demand for the NOS will be driven by practitioners such as physicians and speech pathologists who recommend the device to appropriate subjects. There are several ways in which NSI will work to raise awareness amongst these professionals and the public.
Publicity: Conversational level speech from the first patient will generate much interest in the technology.
A documentary on Phil Kennedy’s work has premiered at Tribeca in summer 2021. Called “The Father of the Cyborgs” it describes my research from the beginning up to the present. It is produced by David Burke, an award-winning documentarian. Also important is a book just published called “Unlocking Erik: A freedom journey to restore speech to those with the locked-in syndrome”. The publishers are Nova Science Publishers. It is available on their web site and on Amazon. It describes the science behind the research as well as contributions from other scientists in the field, a conversation with Erik’s father, and the difficult decision making.
We also plan to work through organizations, such as the LiSA (Locked-in syndrome association), the ALS association, the ALIS (Association for locked-in people in France) to raise awareness of the NOS which is especially important for all those who progress to the completely locked-in state.
Since 1999, NSI has received much publicity regarding the technology being used in the NOS as well as the speech prosthetic. Recently, magazines such as Esquire, Discover and Scientific American Mind have described it in detail. We will leverage these contacts to obtain wide exposure in industry media in which press releases, featured articles and product reviews will help over-all industry and public awareness.
4.4
Pricing
NSI envisages that there will be two kinds of subjects for the Speech prosthetic, paying subjects and subjects with insurance. The early adopters will be exclusively paying subjects while insurance approval for the speech prosthetic is obtained.
Early adopters will pay five hundred thousand dollars for the Speech prosthetic. This will provide income at the early stages and for as long as the market will bear the high price. It will eventually come down.
Eventually however, for mass acceptance, insurance companies will have to agree with NSI on a fee. The most important insurance company in the USA is the government’s Medicare system.
Once Medicare has approved a new device, other insurance companies usually follow shortly thereafter. However, Medicare will not approve the device until FDA approval has been secured which is one reason we need to get a new FDA IDE.
The fee the insurance companies are expected to pay is $140,000 for the reasons discussed in the following paragraphs.
The charge to the insurance companies for implantation and training is based on the economic value of the Speech prosthetic to a typical subject.
According to the Spinal Cord Injury Information Network the lifetime economic impact of being paralyzed (equivalent to being paralyzed and unable to speak) is $5,885,113 per subject. This is based upon a life-time cost of care, $2,924,513, and lost income, $2,960,600.
Assuming that speech restoration allows for recovery of only half the lost income and the price of the NOS is $140,000, the lifetime savings to the subject would be about $1,500,000. Thus, the cost / benefit ratio is a very favorable 10.7:1 (1,500,000 divided by 140,000), making this an attractive proposition for Medicare and other payers. A ratio of 10:1 is the basis for all agreements with Medicare, so any ratio above 10 is very acceptable to Medicare and, hence, other insurance companies.
4.5
ICD Codes
The World Health Organization (WHO) has developed alphanumeric designations for every kind of diagnosis, description of symptoms and causes of death in humans. These are classified in a series of codes called ICD (international classification of diseases) codes.
In the USA, the NCHS (National Center for Health Statistics), a branch of CMS (Centers for Medicare and Medicaid Services) oversees all changes and modifications to the ICD codes, in cooperation with the WHO. The current version of the ICD codes is ICD10.
Medicare pays for a medical device or procedure when a request for payment includes an acceptable code. The ICD codes for access devices (2510) and speech generation (2599) have already been established. Thus, codes for payment for NSI’s devices and prostheses already exist.
4.6
Price-cost Analysis
The total expected reimbursement from Medicare or other insurers for the NOS is $140,000.
This is analyzed as follows:
-
- US$60,000 will be paid by Medicare for the assembled components as shown in Table-3 (which also indicates the relevant ICD codes used by other manufacturers).
-
- Medicare will also pay $80,000 for the implant and training as shown in Table-4 (which also indicates the relevant ICD codes).
-
- The wireless implantable device and other components will cost $20,000 and other components will cost $15,000
-
- Surgery fees (including the fees for the surgeons and other staff as well as the costs of the operating theatre etc.) will be $50,000 as shown in Table-4.
- Standalone training with supervision from NSI will cost $10,000 (Table-4).
Table-3
| Medicare Coverage of NOS components | ||||
| Component | NSI Costs | Insurance Cover | Precedent | ICD10 Code |
| Electrodes | $4000 | $10,000 | Cyberonics 102 Generator | 2599 |
| Implantable Electronics | $15,000 | $20,000 | Cyberonics Systems | 2599 |
| Receiver and Computer | $2,000 | $10,000 | Cyberonics Systems | 2599 |
| Software | $3,000 | $10,000 | Medicare pays for speech language therapists. The software is an integral part of the training that a speech language therapist would do with the subject. This would be similar to Cyberonics’ VNS systems or Medtronic’s DBS systems. | |
| Application Software Decoding Programs | $1,000 | $10,000 | Payer reimburses for the proprietary software that connects to programs that access the Internet, control the environment, music, TV, help or call buttons, etc. | 2510 |
| Total | 25,000 | $60,000 | ||
Table-4
| Medicare Coverage of NOS costs | ||||
| Component | NSI
Costs |
Insurance Cover | Precedent | ICD9 Code |
| Surgery | $50,000 | $60,000 | Deep Brain Stimulation (Medtronics), Cochlear Implants (Boston Scientific), Vagal Nerve Stimulation (Cyberonics) | 2599 |
| Training | $10,000 | $20,000 | Medicare pays for speech language therapists. This would be similar to Cyberonics’ VNS systems or Medtronic’s DBS systems. | 2510 |
| Total | $60,000 | $80,000 | ||
Where NSI performs its own implants (in Belize), total insurance cover will be US$80,000 (components, surgery and training) which will generate a profit contribution of US$20,000 towards the speech prosthetic assembly costs, administrative overheads and net profit (Table 4). The component costs of only $20,000 will generate further profit of $40,000 (table 3) for a total profit of $60,000.
Private subjects will pay substantially more for an implantation, ie., $250,000 during the first year of commercial implants. Thus, the profit contribution per private subjects will be substantially more during the early years.
Where an implant is performed by a BIC other than Belize, it will also have in-house surgery costs limited to $60,000. Neural Signals will have $80,000 revenue which will pay for the components and training.
4.7
Number of Implants
The projections in the table below show the number of brain-implant centers (left-most column) and the number of implants per center through years 1 to 5.
The numbers of trained neurosurgeons will double in Year 3, when commercial implantations begin, and then increase each year thereafter as illustrated in the table.
Table-5: Projected numbers of implants
| BICs | Year-1 | Year-2 | Year-3 | Year-4 | Year-5 |
| 1 Atlanta* | * | * | * | * | * |
| 2 Oxford | 0 | 0 | 4 | 8 | 12 |
| 3 Belize* | 3 | 6 | 16 | 32 | 64 |
| 5 TBN | 0 | 0 | 4 | 8 | 16 |
| 6 TBN | 0 | 0 | 4 | 8 | 16 |
| Total Implants | 3 | 6 | 28 | 64 | 108 |
*Implants for the Atlanta BIC will be performed in Belize City initially. After FDA approval, implants can be performed in Atlanta.
The actual revenues are tabulated in the financial projections (see Section 6.1) and summarized here:
Table 6.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4.8
Production
The Neurotrophic Electrode will be produced by NeuroNexus Inc. It is renamed the NXNE (NeuroNexus Neurotrophic Electrode). Testing has demonstrated 10 times signals than the original in the same dimensional tip. The implantable electronics, when completed and tested will be assembled by our supplier, Tyndall Microelectronics Center, Cork, Ireland. The training software will be developed at NSI. Personnel at NSI will monitor and train the subjects in their homes using remote access facilities.
The monitoring of the remote subjects from NSI will provide an ongoing opportunity to assess quality of the implants and training plus the successful use of the speech prosthetic. This will obviously provide opportunities to correct any problems that might arise.
The number of employees required to assemble each NOS will grow as the demand for the NOS grows, as shown in the table above.
4.9
FDA Approval
Until recently, all implants were performed with permission from the US Food & Drug Administration (FDA), local Human Investigation Committees of the hospitals and Neural Signals Safety committee.
The implantation centers located outside the USA are outside the jurisdiction of the Food and Drug Administration, so these comments do not apply to them.
The FDA recently issued new regulations that must be complied with before implant operations can be performed within the USA. Implementing these requirements is very expensive, as discussed below, and no funding agencies in the USA, such as the National Institutes of Health (NIH), will fund this type of work. Thus it must come from private sources.
In order to implant the devices in subjects within the USA, the suspended FDA IDE (G9600032S) will be need to be closed and a new IDE impplemented. It may seem superfluous to get FDA approval when all implants can be performed in Belize. However, having FDA approval adds a ‘halo’ around the project. It also adds cost since the surgery will cost about $100,000 based on prior experience. Hence, if the cost cannot be negotiated, the cost of the implants may rise.
There are four phases of FDA approval for a new medical device:
-
- Phase One: Demonstrate safety of device. Status: Completed.
-
- Phase Two: Demonstrate efficacy and safety. Status: Completed.
-
- Phase Three: On hold. Pre-market approval, prove safety and efficacy over large number of subjects.
- Post Market Monitoring: After commercial release of the product, there is a post-market monitoring phase where the product is monitored to make sure it has no unexpected side effects, and that efficacy is maintained. This can easily be monitored by NSI personnel who will be in touch with all subjects after implantation.
Neural Signals is in Phase Three for the development of the NOS as a speech prosthesis for G960032S. The FDA wants to close out this IDE. We want to open another IDE. To our advantage, even if implanting abroad, the FDA personnel informed me that if we document the implantation procedures in the detail required by the FDA the data with respect to the implants may be acceptable to the FDA.
To work around this problem in the short term, we will not implant within the FDA’s jurisdiction. Rather, we will implant abroad such as in Belize City. We performed such implants successfully in Belize in 2013 and 2014.
-
Staff & Management
Staff requirements are described in the notes to the financial projections.
-
Executive Management
Dr Phil Kennedy – Founder, chief scientist and acting CEO. His resume is attached in Appendix 6.
During the second year of product development NSI will begin the process of hiring a new CEO, CFO and COO to be chosen according to the criteria required to take a medical device company from the ground up to a mature private or public healthcare corporation.
-
Collaborative research
Collaborative research has been and continues to be a hallmark of the development of technology and related techniques at NSI.
NSI has collaborated with professors and individuals in the Georgia Institute of Technology (Atlanta), Boston University (Massachusetts), MIT (Massachusetts), Northwestern University (Chicago), Rush Medical Center (Chicago), and the University of Pennsylvania, among others.
-
Research Team
NSI has built up close professional relations with a wide range of individuals in the technologies it employs and who cooperate with NSI as required. The core research team includes:
-
- Chief Scientist Phil Kennedy MD PhD … team leader
-
- Dinal Andreasen MSEE … electrical engineer, Georgia Tech
-
- Ryann Williams … software engineer
-
- Jeff Duke … software engineer
-
- Dr. Andre Joel Cervantes … neurosurgeon in Belize
-
- Dr. Princewill Ehirim … neurosurgeon in Atlanta
-
- Dr. Daniel O’Hare … electrical engineer in Tyndall
-
- Dr. Ben Griffin … electrical engineer in Tyndall
-
- Prof. Madeleine Lowery … Biomedical engineer UCD
-
- Prarthana Sakia … Biomedical engineer UCD
- Dr. Jamie Hetke … Electrical engineer NeuroNexus Inc.
-
Previous Research Consultants
Several senior experts have been cooperating with Dr Phil Kennedy and NSI on a long-term basis as opportunities arise. These include:
-
- Frank Guenther PhD of Boston University, and Jon Brumberg PhD of Harvard University, both of whom are experts in speech synthesizers and the modeling of the human speech cortex using functional MRI;
-
- Dr Mark Clements PhD, Georgia Tech, an expert in speech detection algorithms;
-
- Dr Hui Mao MD, Emory Radiology, a functional MRI specialist for targeting the implant.
-
- Dr Roy Bakay, who implanted the first three subjects and all monkeys (He died 2014).
- Dr Andre Cervantes, a neurosurgeon, Belize City, Belize, Central America, who has already participated in two brain implants with Drs Bakay and Kennedy;
-
Investigational Review Board
TBD
-
Data Safety and Monitoring Committee
TBD
-
Business Advisor
- Sal Massaro, BS, Investment Banker, EGL Ventures Inc
-
Legal Advisory Board
-
- Jonathan Sparks, corporate attorney, Sparks Law, Duluth, GA
-
- Bryan Bockhop, patent attorney, Bockhop & Associates, Snellville, GA
- Kristin Zielinski Duggan, FDA attorney, Hogan Lovells, Washington DC
-
Scientific Advisory Board
Andrew Jackson, PhD, is Professor of Neural Interfaces and a Wellcome Trust Senior Research Fellow at the Institute of Neuroscience, Newcastle University, UK. After studying at Oxford University (Physics) and University College London (Neuroscience), Andrew worked for four years with Prof Eberhard Fetz at the University of Washington, Seattle, developing new technologies for bidirectional brain interfaces. Since 2006 he has led a laboratory at Newcastle addressing fundamental scientific questions relating to the control of movement, neural dynamics, sleep and learning. This basic research informs the development of new closed-loop neural interface therapies for conditions including stroke, spinal cord injury and epilepsy.
Thomas Wichmann, MD, received medical training in Germany, followed by postdoctoral training in pharmacology and electrophysiology at the University of Freiburg (Germany) and Johns Hopkins University (Baltimore, MD). In the early 1990s, he completed residency training in Neurology at Emory University (Atlanta, GA), and has been a professor in the movement disorder division in the Department of Neurology at the same University since then, specializing in research and treatment of Parkinson’s disease and Huntington’s disease. His research focuses on brain activity changes that are associated with Parkinson’s disease, and the effects of DBS on brain networks. His work has been continuously funded by NIH and private foundations, and has been published in high-impact journals, such as Science, Brain, Nature and Journal of Neuroscience.
Andre Joel Cervantes, MD, trained in medicine and neurosurgery at the National Autonomous University of Mexico (UNAM) in Mexico City. He now practices neurosurgery in Belize City, Belize and in Mexico. Areas of special interest include Functional, Stereotactic, Radio and Spine Surgery. He co-founded the Belize International Institute of Neuroscience, he founded the Neurosurgical and Spinal Services Associates and is president of the Belize Society of Neurological Surgeons. He has co-authored several scientific papers.
Harvey Wiggin, CEO of Plexon Inc. Harvey attended North Texas State University and obtained a BA in Math and Physics in 1964. In 1968 he graduated from SMU with an MS Electrical Engineering degree with minors in Biomedical Engineering and Computer Science. After a year at Nuclear Chicago Corp developing system software he worked until 1983 at the Callier Center for Communication Disorders in Dallas with neuroscientists studying the auditory nervous system. In 1983 Harvey started Spectrum Scientific as a small company to develop and provide computer-based instrumentation for research into the brain and nervous system. The company slowly grew, incorporating along the way as Plexon Inc. Plexon presently employs 25 people and is the oldest and most experienced company in this research area.
-
Funding & Financial Projections
To-date NSI Inc has been funded by non-refundable SBIR grants from the US government’s National Institutes of Health (NIH) amounting to more than $4.4 million in recent years and from Dr. Kennedy’s personal funds of $450,000.00
The Small Business Innovation Research (SBIR) program is a United States Government program, coordinated by the Small Business Administration, in which 2.5% of the total extramural research budgets of all federal agencies with extramural research budgets in excess of $100 million are reserved for contracts or grants to small businesses, in order to spur technological development in the small business sector.
As the company has now reached the stage where it is commercializing the NOS for speech restoration, further funding from the NIH is not possible. In addition, NSI is no longer inclined to pursue grants from government agencies since too much time is wasted between the inception of an idea and funding – typically two years – and the funding is inadequate. We feel that only private funding will provide the finance necessary to commercialize this product.
This first round funding request is for US$1,000,000 equity for a 10% ownership of ordinary stock of Neural Signals Inc. Pre-funding valuation is $4,500,000, post funding will be $10,000,000. The first round is for two years.
-
Financial Projections
Financial projections, projected balance sheets, profits & loss accounts, and cash flow forecasts will be available. Table 6 outlines the revenues.
| BALANCE SHEETS | ||||||||||
| Notes | As at last day of year | Yr-1 | Yr-2 | Yr-3 | Yr-4 | Yr-5 | ||||
| US$ | US$ | US$ | US$ | US$ | ||||||
| Fixed Assets | ||||||||||
| 1 | BICs set-up costs | 0 | 0 | 100,000 | 300,000 | 700,000 | ||||
| Depreciation | 0 | 0 | 20,000 | 80,000 | 220,000 | |||||
| Net book values | 0 | 0 | 80,000 | 220,000 | 480,000 | |||||
| Current Assets | ||||||||||
| Receivables | 0 | |||||||||
| Bank | 41,438 | 75,863 | 915,143 | 1,824,638 | 4,249,557 | |||||
| Total current assets | 41,438 | 75,863 | 915,143 | 1,824,638 | 4,249,557 | |||||
| Current Liabilities | ||||||||||
| Corporate taxes due | 0 | |||||||||
| Accounts Payable | 0 | |||||||||
| Loans from shareholders | 0 | 0 | ||||||||
| Net cash & bank | 0 | |||||||||
| Total current liabilities | 0 | 0 | 0 | 0 | 0 | |||||
| Net Current Assets | 41,438 | 75,863 | 915,143 | 1,824,638 | 4,249,557 | |||||
| Net Assets | 41,438 | 75,863 | 995,143 | 2,044,638 | 4,729,557 | |||||
| Financed by: | ||||||||||
| Equity | ||||||||||
| Common stock 85% | 0 | |||||||||
| New capital bfw 15% | 500,000 | 1,000,000 | 1,000,000 | 1,000,000 | ||||||
| New Capital 15% | 500,000 | 1,000,000 | ||||||||
| Total paid-up capital | 500,000 | 1,500,000 | 1,000,000 | 1,000,000 | 1,000,000 | |||||
| Retained earnings | ||||||||||
| At start of year | 0 | -488,563 | -954,138 | -34,858 | 1,014,637 | |||||
| Profit (-Loss) for year | -488,563 | -465,575 | 919,280 | 1,049,494 | 2,684,919 | |||||
| At end of year | -488,563 | -954,138 | -34,858 | 1,014,637 | 3,699,556 | |||||
| Total shareholders | 11,438 | 545,863 | 965,143 | 2,014,637 | 4,699,556 | |||||
| Long-term liabilities | ||||||||||
| Capital injection | 0 | |||||||||
| Loans from s’holders | 30,000 | 30,000 | 30,000 | 30,000 | 30,000 | |||||
| Total long-term liabs | 30,000 | 30,000 | 30,000 | 30,000 | 30,000 | |||||
| Total long-term | ||||||||||
| financing | 41,438 | 575,863 | 995,143 | 2,044,637 | 4,729,556 | |||||
| diffs | 0 | -500,000 | 0 | 1 | 2 | |||||
| 1 | FIXED ASSETS | |||||||||
| The only fixed assets are NSI’s investment in the Brain Implantation Centers (BICs) | ||||||||||
| NSI will pay each BIC $100,000 to help with the setup of implantation centers. | ||||||||||
| This will not be refunded by the BICs and is there being treated as an investment | ||||||||||
| in the books of NSI. It is being depreciated at 20% per annum on a straight-line basis. | ||||||||||
| PROFIT & LOSS ACCOUNTS (Forecast) | ||||||||||
| For the years | Yr-1 | Yr-2 | Yr-3 | Yr-4 | Yr-5 | |||||
| US$ | US$ | US$ | US$ | US$ | ||||||
| Income | ||||||||||
| Number of implants | ||||||||||
| NSI in Belize | 1 | 1 | 4 | 8 | 16 | |||||
| BICs | 0 | 2 | 4 | 6 | ||||||
| Total number of implants | 1 | 1 | 6 | 12 | 22 | |||||
| Implant income for NSI | ||||||||||
| NSI implants | 2,000,000 | 2,800,000 | 5,600,000 | |||||||
| BIC implants | 100,000 | 200,000 | 300,000 | |||||||
| Total implant income | 0 | 0 | 2,100,000 | 3,000,000 | 5,900,000 | |||||
| Cost of implants | ||||||||||
| Cost of implants – NSI | 251,400 | 502,800 | 1,005,600 | |||||||
| Cost of implants – BICs | 64,000 | 128,000 | 192,000 | |||||||
| Components (all) | 37,000 | 10,000 | ||||||||
| Surgeries (NSI only) | 40,000 | 40,000 | ||||||||
| Animal Implant | 0 | |||||||||
| Speech training (NSI only) | ||||||||||
| Total cost of implants | 77,000 | 50,000 | 315,400 | 630,800 | 1,197,600 | |||||
| Gross profit – implants | -77,000 | -50,000 | 1,784,600 | 2,369,200 | 4,702,400 | |||||
| Net Income | -77,000 | -50,000 | 1,784,600 | 2,369,200 | 4,702,400 | |||||
| Expenses | ||||||||||
| Payroll expense | 300,000 | 314,760 | 700,000 | 740,000 | 740,000 | |||||
| Contractual costs – Georgia Tech | 0 | 0 | 0 | |||||||
| Facilities and administration | 97,713 | 93,115 | 18,698 | 18,698 | 18,698 | |||||
| Insurance | 25,852 | 25,852 | 25,852 | |||||||
| Professional fees | 18,827 | 18,827 | 18,827 | |||||||
| Licences and permits | 732 | 732 | 732 | |||||||
| Marketing | 10,000 | 10,000 | 10,000 | |||||||
| Rent | 18,000 | 18,000 | 18,000 | |||||||
| Telephone | 1,892 | 1,892 | 1,892 | |||||||
| Travel & Education | 6,700 | 6,700 | 10,101 | 10,101 | 10,101 | |||||
| Utilities | 3,323 | 2,432 | 2,432 | |||||||
| Financial services charges | 0 | 0 | 0 | |||||||
| Taxes | 5,000 | 5,000 | 5,000 | |||||||
| Dues and subscriptions | 3,557 | 3,557 | 3,557 | |||||||
| Postage and deliveries | 1,655 | 1,655 | 1,655 | |||||||
| Printing and reproductions | 379 | 379 | 379 | |||||||
| Supplies | 26,170 | 26,170 | 26,170 | |||||||
| Repairs | 1,134 | 1,134 | 1,134 | |||||||
| Depreciation | 20,000 | 60,000 | 140,000 | |||||||
| Sundries | 1,000 | |||||||||
| Total Expenses | 404,413 | 415,575 | 865,320 | 944,428 | 1,024,428 | |||||
| check | ||||||||||
| Net Profit (-Loss) before tax | -481,413 | -465,575 | 919,280 | 1,424,772 | 3,677,972 | |||||
| check | -488,563 | -465,575 | 3,677,971 | |||||||
| Corporation tax | 0 | 375,277 | 993,052 | |||||||
| Net profit after tax | 919,280 | 1,049,495 | 2,684,920 | |||||||
| Payroll | ||||||||||
| Numbers | ||||||||||
| CEO – 180k | 1 | 1 | 1 | |||||||
| Chief Scientist – 180k | 6 mos | 6 mos | 1 | 1 | 1 | |||||
| Programmer – 100k | 1 | 1 | 1 | |||||||
| Biomedical Engineer – 100k | 1 | 1 | 0 | 0 | 0 | |||||
| Lab Assistant – 40k | 1 | 2 | 2 | |||||||
| Speech Language Pathologist – 60k | 0 | 1 | 2 | |||||||
| Office Manager – 40k | 1 | 1 | 1 | |||||||
| Implant Coordinator – 60k | 1 | 1 | 1 | |||||||
| 1 mo | 1 mo | |||||||||
| Costs | $ | $ | $ | |||||||
| CEO – 180k | 180,000 | 180,000 | 180,000 | |||||||
| Chief Scientist – 180k | 60,000 | 60,000 | 180,000 | 180,000 | 180,000 | |||||
| Programmer – 75k | 100,000 | 100,000 | 100,000 | |||||||
| Biomedical Engineer – 60k | 108,000 | 113,760 | 100,000 | 100,000 | 100,000 | |||||
| Lab Assistant – 40k | 40,000 | 80,000 | 80,000 | |||||||
| Office Manager – 40k | 40,000 | 40,000 | 40,000 | |||||||
| Implant Coordinator – 60k | 60,000 | 60,000 | 60,000 | |||||||
| Bookkeeper – 60k | 6,000 | 6,000 | ||||||||
| 174,000 | 179,760 | 700,000 | 740,000 | 740,000 | ||||||
| Corporation tax are based on the unadjusted profits before tax | ||||||||||
| Rates used … 21% Federal tax rate … 6% Georgia tax rate | ||||||||||
| It is assumed for cash flow purposes that corporation taxes are paid on the last day of the year | ||||||||||
| to which they relate | ||||||||||
| CASH FLOW FORECASTS | Yr-1 | Yr-2 | Yr-3 | Yr-4 | Yr-5 | ||||
| US$ | US$ | US$ | US$ | US$ | |||||
| Opening Cash | 41,438 | 75,863 | 915,143 | 1,824,638 | |||||
| Cash Inflows | |||||||||
| Implants – NSI | 2,000,000 | 2,800,000 | 5,600,000 | ||||||
| Implants – from BICs | 100,000 | 200,000 | 300,000 | ||||||
| Loan from shareholder – PRK | 30,000 | ||||||||
| Equity funding | 500,000 | 500,000 | |||||||
| Total inflows | 530,000 | 500,000 | 2,100,000 | 3,000,000 | 5,900,000 | ||||
| Total available | 530,000 | 541,438 | 2,175,863 | 3,915,143 | 7,724,638 | ||||
| Cash Outflows | |||||||||
| Components-NSI | 34,150 | ||||||||
| Materials+Supplies | 10,000 | 10,000 | |||||||
| Surgery fees | 40,000 | 40,000 | |||||||
| Animal implant | 0 | ||||||||
| Facilities+Admin | 97,713 | 93,115 | |||||||
| Costs of implants | 315,400 | 630,800 | 1,197,600 | ||||||
| Payroll | 300,000 | 314,760 | 700,000 | 740,000 | 740,000 | ||||
| Contractual costs – Georgia Tech | 0 | 0 | 0 | ||||||
| Facilities and administration | 18,698 | 18,698 | 18,698 | ||||||
| Insurance | 25,852 | 25,852 | 25,852 | ||||||
| Professional fees | 18,827 | 18,827 | 18,827 | ||||||
| Licences and permits | 732 | 732 | 732 | ||||||
| Marketing | 10,000 | 10,000 | 10,000 | ||||||
| Rent | 18,000 | 18,000 | 18,000 | ||||||
| Telephone | 1,892 | 1,892 | 1,892 | ||||||
| Travel, Educat’n & Entermt | 6,700 | 6,700 | 10,101 | 10,101 | 10,101 | ||||
| Utilities | 3,323 | 2,432 | 2,432 | ||||||
| Financial services charges | 0 | 0 | 0 | ||||||
| Taxes | 5,000 | 5,000 | 5,000 | ||||||
| Dues and subscriptions | 3,557 | 3,557 | 3,557 | ||||||
| Postage and deliveries | 1,655 | 1,655 | 1,655 | ||||||
| Printing and reproductions | 1,000 | 379 | 379 | 379 | |||||
| Supplies | 26,170 | 26,170 | 26,170 | ||||||
| Repairs | 1,134 | 1,134 | 1,134 | ||||||
| BIC set-ups | 100,000 | 200,000 | 400,000 | ||||||
| Corporation tax paid | 0 | 375,277 | 993,052 | ||||||
| Total Outflows | 488,563 | 465,575 | 1,260,720 | 2,090,505 | 3,475,080 | ||||
| Closing Cash | 41,438 | 75,863 | 915,143 | 1,824,638 | 4,249,557 | ||||
| 41,438 | 75,863 | 915,143 | 1,824,638 | 4,249,557 |
Contact Details
For further details, or to explore an investment opportunity further, please contact:
Philip Kennedy, MD, PhD
Neural Signals Inc
3400 McClure Bridge Road, Suite D402,
Duluth, Georgia 30096, USA
Mobile: (+1) 404-771-2034
Email: phlkennedy@aol.com
Web site: www.neuralsignals.com
References
1] Kennedy PR, Mirra S and Bakay RAE. The Cone Electrode: Ultrastructural Studies Following Long-Term Recording Neuroscience Letters, 142:89-94, (1992a).
2] Gearin M and Kennedy PR. Histological confirmation of myelinated neural filaments within the tip of the Neurotrophic Electrode after a decade of neural recordings. Front. Hum. Neurosci. 21 April 2020 https://doi.org/10.3389/fnhum.2020.00111.
3] Kennedy P.R., Dinal S. Andreasen, Jess Bartels, Princewill Ehirim, E. Joe Wright, Steven Seibert, Andre Joel Cervantes. Validation of Neurotrophic Electrode long-term recordings in human cortex. Handbook of Biomedical Engineering. 2017.
4] Kennedy PR, Bakay RAE and Sharpe SM. Behavioral correlates of action potentials recorded chronically inside the Cone Electrode. NeuroReport, 3:605-608, (1992b).
5] Kennedy PR and King B. Dynamic interplay of neural signals during the emergence of cursor related cursor in a human implanted with the Neurotrophic electrode. CH 7 in Neural Prostheses for Restoration Spee
6] Sponheim C, Papadouakis V, Collinger JL, Downey J, Weiss J, Pentousi L, Elliott K, Hatsopoulos NG. Longevity and reliability of chronic unit recordings using the Utah, intracortical multi-electrode array. J. Neural Eng., 2021, Dec 28; 18(6):10.1088/1741-2552.
7] Downey JE, Schwed N, Chase SM, Schwartz AB, Collinger JL. Intracortical recording stability in human brain-computer interface users. J Neural Eng. 2018, 15(4):046016.
7a] Dagenhart AD, Eles J, Dum R, Mischel JL, Smalianchuk I, Endler B et al. Histological evaluation of a chronically implanted electrocorticographic electrode grid in a non-human primate. J Neural Eng, 2016, 13(4):046019. Doi 10.1088/1741-2560/13/4/046019.
8] Stavisky SD, Willett FR, Wilson GH, Murphy BA, Rezaii P, Avansino DT, Memberg WD, Miller JP, Kirsch RF, Hochberg LR, Ajiboye AB, Druckmann S, Shenoy KV, Henderson JM. Neural ensemble dynamics in dorsal motor cortex during speech in people with paralysis. Elife. 2019 Dec 10;8:e46015. doi: 10.7554/eLife.46015.
9] An Integrated Brain-Machine Interface Platform With Thousands of Channels. Musk E; Neuralink. J Med Internet Res. 2019 Oct 31;21(10):e16194. doi: 10.2196/16194.
10] Steinmetz NA, Aydin C, Lebedev A, Okun M, Pachitariu M, Bauza M, Beau M, Bhagat J, Böhm C, Broux M, Chen S, Colonell J, Gardner RJ, Karsh B, Kloosterman F, Kostadinov D, Mora-Lopez C, O’Callaghan J, Park J, Putzeys J, Sauerbrei B, van Daal RJJ, Vollan AZ, Wang S, Welkenhuysen M, Ye Z, Dudman JT, Dutta B, Hantman AW, Harris KD, Lee AK, Moser EI, O’Keefe J, Renart A, Svoboda K, Häusser M, Haesler S, Carandini M, Harris TD. Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science. 2021 Apr 16;372(6539):eabf4588. doi: 10.1126/science.abf4588.
11] Neely RM, Piech DK, Santacruz SR, Maharbiz MM, Carmena JM. Recent advances in neural dust: towards a neural interface platform. Curr Opin Neurobiol. 2018 Jun;50:64-71. doi: 10.1016/j.conb.2017.12.010. Epub 2018 Jan 11. PMID: 29331738
12] Kennedy PR and Bakay RAE. Restoration of neural output from a paralyzed patient using a direct brain connection. NeuroReport 9,1707-11, 1998.
13] Kennedy PR, Dinal Andeasen, Brandon King, Todd Kirby, Hui Mao, Melody Moore, Princewill Ehirim. Correlations between human motor cortical local field potentials, action potentials, contralateral arm EMG activity and digit movements. J. Neural Engineering 2011;194:1-25. doi: 10.1016/B978-0-444-53815-4.00020-0. PMID: 21867791.
14] Kennedy PR, Mirra S and Bakay RAE. The Cone Electrode: Ultrastructural Studies Following Long-Term Recording Neuroscience Letters, 142:89-94, (1992a).
15] Kennedy P.R., Gambrell C, Ehirim P, and Cervantes A. Advances in the development of a speech prosthesis. Book chapter in Brain-Machine Interfaces: Uses and Developments accepted 2017.
Appendices
- Refereed Publications
- Gearin M and Kennedy PR. Histological confirmation of myelinated neural filaments within the tip of the Neurotrophic Electrode after a decade of neural recordings. Frontiers in Human Neuroscience. 2020 https://doi.org/10.3389/fnhum.2020.00111.
- Kennedy PR. To invade or not to invade, that is the question for brain computer interfacing. Current Trends in Neurology, 12:31-38, 2018.
- Kennedy P.R., Gambrell C, Ehirim P, and Cervantes A. Advances in the development of a speech prosthesis. Book chapter in Brain-Machine Interfaces: Uses and Developments accepted 2017.
- Degenhart AD, Eles J, Dum R et al. Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate. J. Neural Eng. 2016, 13:046019. doi: 10.1088/1741-2560/13/4/046019
- Kennedy P.R., Dinal S. Andreasen, Jess Bartels, Princewill Ehirim, E. Joe Wright, Steven Seibert, Andre Joel Cervantes. Validation of Neurotrophic Electrode long-term recordings in human cortex. Handbook of Biomedical Engineering. 2017.
- Kennedy P.R. Brain-machine interfaces as a challenge to the “moment of singularity”. Front Syst Neurosci. 2014 Dec 17;8:213.
- Sarmah E. and Kennedy P.R. Detecting Silent Vocalizations in a Locked-In Subject. Neuroscience Journal, Volume 2013 (2013), Article ID 594624.
- Kennedy, P.R., AndreasenD.S., Bartels, J., EhirimP., MaoH., VellisteM.,WichmannT.,Wright E.J. (2011) Making the lifetime connection between brain and machine for restoring and enhancing function. Proceedings in Brain Research, Ch.1, August 2011.
- Brumberg J., Wright EJ, Andersen D, Guenther FH and Kennedy PR. Classification of intended phoneme production from chronic intracortical microelectrode recordings in speech motor cortex. 2011. Frontiers in Neuroscience 5(65)1-14.
- Brumberg JS, Nieto-Castanon A, Kennedy PR, Guenther FH. Brain-Computer Interfaces for Speech Communication. Speech Commun. 2010 Apr 1;52(4):367-379.
- Kennedy, PR. Changes in emotional state modulate neuronal firing rates of human speech motor cortex: A case study in long-term recording. Neurocase 2011 17(5), 381-393.
- Guenther, F.H., Brumberg, J.S., Wright, E.J., Nieto-Castanon, A., Tourville, J.A., Panko, M., Law, R., Siebert, S.A., Bartels, J.L., Andreasen, D.S., Ehirim, P., Mao, H., and Kennedy, P.R. A wireless brain-machine interface for real-time speech synthesis. PLoS ONE. (2009) 9;4(12):e8218
- Neurotrophic electrode: Method of assembly and implantation into human motor speech cortex. Bartels J, Andreasen D, Ehirim P, Mao H, Seibert S, Wright EJ, Kennedy PR. J Neurosci Methods. 2008 Sep 30;174(2):168-76. Epub 2008 Jul 10.
- Comparing Electrodes for use as Cortical Control Signals: Tiny Tines, Tiny Wires or Tiny Cones on Wires: Which is best? Kennedy PR. The Biomedical Engineering Handbook, Third Edition. Ed.: Joe Brazino, 32-1 to 32-14, 2006, revised 2011.
- Using Human Extra-cortical Local Field Potentials to Control a Switch. Kennedy PR, DinalAndreasen, PrincewillEhirim, Brandon King, Todd Kirby, Hui Mao, Melody Moore. J. Neural Eng.1: 72-77, 2004.
- Computer Control Using Human Cortical Local Field Potentials. Kennedy PR, Kirby MT, Moore MM, King B & Mallory A. IEEE Trans on Neural Systems and Rehabilitation Eng. 12(3), 339-344, 2004.
- A Decision Tree for Brain-Computer Interface Devices. P.R.KennedyandK.Adams, IEEE Trans Biomed. Eng, accepted, 2003.
- Dynamic interplay of neural signals during the emergence of cursor related cursor in a human implanted with the Neurotrophic electrode. Kennedy PR and King B. CH 7 in Neural Prostheses for Restoration of Sensory and Motor Function. Eds. Chapin J and Moxon, K. CRC Press, 2001.
- Direct control of a computer from the human central nervous system. Kennedy PR, Bakay RAE, Adams K, Goldthwaite J, and M. Moore. IEEE Trans. Rehab. Eng., 8(2), 198-202, 2000.
- Restoration of neural output from a paralyzed subjectsubject by a direct brain connection. P.R.Kennedy&R.A.E.Bakay, NeuroReport, 9:1707-11, 1998.
- Activity of single action potentials in monkey motor cortex during long-term task learning. P.R. Kennedy& R.A.E. Bakay. Brain Research, 760, 251-4, 1997.
- Behavioral correlates of action potentials recorded chronically inside the Cone Electrode. P.R. Kennedy, R.A.E. Bakay and S.M. Sharpe. NeuroReport, 3:605-608, (1992).
- The Cone Electrode: Ultrastructural Studies Following Long-Term Recording. P.R. Kennedy, S.Mirra and R.A.E. Bakay. Neuroscience Letters, 142:89-94, (1992).
Patent list:
-
- “Implantable Neural Electrode.” Patent #: 4,852,573, issued on August 1, 1989.
-
- “System and Method for Speech Generation from Brain Activity.” Serial number: 1/007,380. Filing date: 12/08/2003. Issued September 2007.
-
- Apparatus and method for detecting neural signals and using neural signals to drive external functions. Issued March 6, 2007. US: 7,187,967.B2
-
-
Medication Dispensing Device. Filed 5/29/2009. Issued August 30, 2011. US 8,009,040.B2
-
-
-
“Quantum Dot Neurotrophic Electrode Arrays”. Filed 3/6/ 2007. Serial Number 60/893,16. Foreign (PTC) filing: Neurotrophic Electrode Neural Interface Employing Quantum Dots.” Filing date: 3/5/2008. Serial Number 12042742.
-
-
-
Neurotrophic Electrode System. Filed 12/15/2016. Serial number 15/380,097
-
-
- “Neural Electrode Array.” Serial #: 11/096,897. Filing date: 04/01/2005.
-
- “Software controlled electromyogram control system”. Serial #: 10/881,923. Filing date: 06/30/2004.
-
- “Detecting Neural Signals and using same to drive external functions.” Serial #: 10/675,703. Filing date: 9/30/2003.
-
- “Speech Prosthesis Employing Beta Peak Detection”; Serial number: 15/800,589 Filed November 2017.
- “Silent speech and silent listening” Submitted 2021.
- Abstract from the Cyberkinetics Project.
| Program#/Poster#: | 84.23/RR17 |
| Presentation Title: | Use of the BrainGate Neural Interface System for more than five years by a woman with tetraplegia |
| Location: | Hall A-C |
| Presentation time: | Saturday, Nov 12, 2011, 3:00 PM – 4:00 PM |
| Authors: | *L. R. HOCHBERG1,3,6,7,8, D. BACHER3, L. BAREFOOT1, E. BERHANU1, M. J. BLACK4,9, S. S. CASH1,8, J. M. FELDMAN5, E. M. GALLIVAN1, M. HOMER3, B. JAROSIEWICZ5,6, B. KING5, J. LIU3, W. Q. MALIK2,8, N. Y. MASSE5, J. A. PERGE3, D. M. ROSLER6,3,1, N. SCHMANSKY1, J. D. SIMERAL6,3,1, B. TRAVERS5, W. TRUCCOLO5, J. P. DONOGHUE5,6; 1Neurol., 2Anesthesiol., Massachusetts Gen. Hosp., Boston, MA; 3Engin., 4Computer Sci., 5Neurosci., Brown Univ., Providence, RI; 6Rehabil. R&D Service, VA Med. Ctr., Providence, RI; 7Neurol., Brigham & Women’s and Spaulding Rehabil. Hosp., Boston, MA; 8Harvard Med. Sch., Boston, MA; 9Max Planck Inst. for Intelligent Systems, Tuebingen, Germany |
| Abstract: | Intracortically-based neural interfaces have the potential to restore communication, mobility, and independence for people with spinal cord injury, brainstem stroke, neuromuscular disease, or limb loss. The ongoing pilot clinical trials of the investigational BrainGate Neural Interface System (IDE) have enrolled five participants to date, collecting initial clinical experience in the use of a chronic intracortical neural interface system that can record single unit action potentials, multi-unit activity, and local field potentials simultaneously. Recently, one participant with tetraplegia and anarthria due to brainstem stroke surpassed five years of participation in the clinical trial using a single array of 96 microelectrodes placed in the dominant precentralgyrus. Through her last week of scheduled research sessions (prior to voluntary, pre-planned trial exit), she was able to use the BrainGate system for controlling a prosthetic limb which she had never before used, as well as an efficient point-and-click radial speller that permitted her to communicate words and sentences readily. While signal quality was considerably lower than that seen in the months just after array placement, the demonstration of confident and useful neuronal ensemble-based control of virtual and physical devices at nearly 2000 days post-implant provides a benchmark for BCI research. Based in part on the clinical, neuroscientific, and neuroengineering knowledge and experience obtained thus far, the collaborative BrainGate research is moving toward engaging additional pilot clinical trial sites. |
———————————————————————
- Principles of Operation of the Brain Implant Centers
Implantation centers. These centers will assess the subjects for implantation, purchase the electrodes and electronics from NSI along with the license, perform the implantations and then refer the subjects to NSI for training using remote access and training software.
Principles of Operation:
1] Quality is the most important principle of human implantation. To achieve the highest quality, all knowledge and experience must be shared. Thus, a central depository of implantation and training information is essential to maintain quality. This information will be secured according to HIPAA guidelines and stored in NSI headquarters in Duluth, Georgia, USA.
2] Only NSI approved and trained personnel will be allowed to perform implantations and training. Such personnel will be certified and licensed by NSI.
3] All implantations would be according to FDA regulations, the Declaration of Helsinki and all local regulations.
Notes:
NSI can apply for other uses and techniques:
For example, the FDA gave permission to Cyberkinetics Inc to use the Utah Array electrode for movement control. It would likely give permission for NSI to develop movement control of paralyzed muscles and robotic limbs, or full robots. Thus, whereas we may begin with the speech prosthetic, it is unlikely that a limit will be placed on our development.
CURRICULUM VITAE
KENNEDY, Philip R. 2024
Founder and Chief Scientist
Neural Speech, formerly Neural Signals Inc., 3400 McClure Bridge Road, Suite D402, Duluth, Georgia 30096.
Web site: www.neuralsignals.com
Neurology Practice
Neurology Office: 3400 McClure Bridge Road, Duluth, GA 30096.
Dr. Kennedy will go full time with NSI when funded and not practice neurology.
Personal
Born November, 7, 1947
Four children: Dermot, Finian, Naomi and Nash.
Five grandchildren Campbell, Colin, Declan, Hannah and Leah.
Ex-Soccer coach for daughter’s under 10 team
Marathon Runner, Atlanta 1990,’91,’92
Half Marathon, Atlanta 1989,’95
Peachtree Road Race 10K Atlanta, 1989-93,97,98,01,04
Comet Run 10k 2005.
Enjoys chess, photography, guitar
Children’s non-fiction book author, science prediction author
Education
1997 Residency in Neurology at Emory University Med. Sch., Atlanta, GA 30322.
1983 Ph.D. Physiology (Neurophysiology and Neuroanatomy) Northwestern University, Chicago, IL 1976 F.R.C.S.I. (General Surgery Boards) Royal College of Surgeons in Ireland
1972 M.D. National University of Ireland (MB, BCh, BAO)
Honors & Awards:
Church and State Honorary Associate 2019
Researcher of the Year, Neurotechnology Report 2013
Alfred Mann Foundation Award Recipient for Scientific Achievement 2004
Winner of Resource Forum Enterprise Award, January, 2002
Wallace H. Coulter Prize for Innovative Technology, runner up. 2001
Winner of World Technology Award in Health and Medicine 2000
Winner of Discover Magazine Annual Award for Technological Innovation in the Assistive Technology Category 1999
Winner of Atlanta Business Chronicle Health Care Heroes Award in Health Care Innovation category 1999
Life Time Member of National Registry of Who’s Who. 1999
Pharmacia & Upjohn Award for outstanding research in CNS 1997
Parke-Davis Fellow 1994
National Research Council Senior Associateship Award 1992
Alberta Heritage Medical Research Fellowship 1985
Northwestern University Graduate School Scholarship 1980-83
Muscular Dystrophy Association of Canada Fellowship 1979-80
Sheppard Memorial Prize, Ireland 1975
Awards and media publications 1999 to date (partial list):
Wired Magazine 2016, MIT Technology Review 2016.
Experimentarium Radio Interview Copenhagen (2010) Discover Sep 2008, Esquire Sep 2008, Scientific American Mind Oct 2008, Scientific American 2008, CNN (2007), PBS (2006), BBC (2006), Discovery Channel (2006), Discover Magazine Award (1999), Atlanta Magazine’s Health Care Hero’s Award (1999), World Technology Network Award (London, England) (2000), Resource Forum Entrepreneur Award (2001), US News and World Report, Chicago Tribune, USA Today, Boston Globe, Detroit News, Los Angeles Times, The Washington Post, New York Times, Newsday, San Francisco Chronicle, Atlanta Journal-Constitution, The Sciences, Current Science (Weekly Reader), New Scientist, Popular Science, Atlanta Magazine, CBS Evening News, CBS This Morning, ABC World News Tonight, ABC Good Morning America, Dateline NBC, The Learning Channel, Associated Press Television, Discovery (Canada), PBS “Frontiers of Medicine”, Tokyo Broadcasting System, Swedish Television, RTL German TV, Brazilian TV, FOX Network, CNN, BBC World News, Metro Source Radio (Syndicated Radio Broadcast), Associated Press Radio Network, National Public Radio “All Things Considered”, Forbes Magazine, Sunday Times, Creative Loafing.
Documentary due Fall 2020: “The Father of the Cyborgs”
Book published April 2020 by Nova Science Publishers: “Unlocking Erik: A freedom journey to restore speech to those with the locked-in syndrome.”
Professional Affiliations
The Society for Neuroscience
American Academy of Neurology
American Medical Association
American Academy of Sleep Medicine
International Brain Research Organization
IEEE Engineering in Biology and Medicine Society
American Association for the Advancement of Science
American Speech-Language-Hearing Association
Employment History
-
CEO & Chief Scientist Neural Signals Inc (now Neural Speech) 1987 to date
3400 McClure Bridge Road, Building D, Suite B, Duluth, Georgia 30096.
Independent contractor at Dr. Gadlage’s Clinic 2017 to date
Self-employed in Community Neurological Clinic 1997 to 2016
3400 McClure Bridge Road, Building D, Suite A, Duluth, Georgia 30096..
Private Practice with Dr. Evelyn A. Sevilla, MD 1997-98
9217 Park West Blvd., Building A3, Knoxville, TN 39723.
Emory University, Department of Neurology, Atlanta
PGY4 Resident in Neurology 1994-97
Emory University, Department of Medicine, Atlanta
PGY1 Resident in Transitional Year Medicine 1993-94
Georgia Institute of Technology, Atlanta, Ga
Senior Research Scientist, Director, Neuroscience Lab 1990-97
Research Scientist II, Bioengineering Center 1987-90
Research Scientist II, Biomedical Res. Div. 1986-87
Emory University’s Yerkes Primate Center
Collaborative Scientist 1988-97
Emory University Medical School, Atlanta, GA
Research Associate, Dept. Physiology 1983-86
Northwestern University Graduate School, Chicago, IL
Graduate Student, Dept. Physiology 1980-83
Univ. of Western Ontario Med. Sch., London, Ont., Canada
Post-Doctoral Fellow, Dept. Physiology 1978-80
Resident, Dept. of Neurosurgery 1976-78
Royal College of Surgeons in Ireland
Surgical Resident 1972-76
Referee for Funding Agencies:
Committee Member:
National Institute of Health, Small Business proposals to the
Bio-Psychology Study Section of the NINDS. Also, NIMH reviewer
Ad-hoc referee:
National Science Foundation
Neuroscience Letters.
Nature Communications
Nature
J Neural Engineering
Man in Motion Rick Hensen Spinal Cord Research Society
The Easter Seal Research Institute of Ontario
Spinal Cord Research Foundation
Trends in Cognitive Science
IEEE Trans. Biomed. Engineering
IEEE Trans. Rehab. Engineering (Associate Editor)
Journal of Neuroscience Methods
Funding History Total: $4,199,641
- SBIR, NIDCD, Phase 2, Supplement, Brain to Speech Interface
$74,500 2009
- SBIR, NINDS, Phase 1, Polyimide Neurotrophic Electrode: Fabrication and Testing.
$99,850 2009
- SBIR, NIDCD, Phase 2, Brain to Speech Interface
$778,553 2007
- SBIR, NINDS, Phase 2, IC Design and Development, with GA Tech
$750,000 2006
- SBIR, NICDC, Phase 1, Brain to Speech Interface
$100,000 2004
- SBIR, NINDS, Phase 1, EMG development
$100,000 2004
- SBIR, NINDS, Phase 1, IC Design and Development, with GA Tech.
$100,000 2004
- SBIR, NINDS, Phase 1, with Foster Miller and InnerSea Tech
$100,000 2003
- SBIR, NINDS, Phase 1, Miniaturizing Implant Electronics
$100,000 2003
- SBIR, NINDS, Phase 1, Muscle Communication for Computer Control” $100,000 1999
- SBIR, NINDS, Phase 2, “Interfacing with the Human CNS”
$1,115,000 1999
- SBIR, NINDS, Phase 1, “Interfacing with the Human CNS”
$100,000 1998
- Dept. of Neurology, Emory University, support grant to the laboratory, three years. $48,000 1994
- National Research Council Senior Associateship Award funding salary for 6 months to work with Dr. Ed Schmidt of the Laboratory of Neural Control in the NIH.
$24,000 1992
- Emory Univ.-Georgia Tech Biomedical Technology Research Center grant to Dr. Bakay and self to continue development of the Cone Electrode for neural prosthetics.
$29,900 1992
- Collaboration with Veterans Administration, Dr. Kent Davey, on project involving electromagnetic stimulation of muscles and nerves.
$4,700 1992
- Georgia Tech- Med. Coll. Georgia Biomed. Res. Center: Plastic changes in somatosensory cortex of raccoons monitored with a long-term recording system, the Cone Electrode. With Drs. Doetsch and Stoney,
$33,340 1992
- Atlanta-Jewish Federation: Combined development of the Cone Electrode for Neural Prosthetics. With Dr. Roger Nathan of Israel.
$5,000 1992
-
- Center for Rehabilitation Technology: Survey of the area of functional neuromuscular stimulation and biocommunication.
- $5,000 1991
- Third year of NINDS grant on Red Nucleus 1991
- Second year of NINDS grant on Red Nucleus 1990
- American Paralysis Association: Accessing control signals in motor cortex for restoration of movement to paralyzed muscles.
$30,000 1989
- Emory-Georgia Tech Bioengineering Res. Ctr.
$29,000 1989
- Structure and Connections of Rat Red Nucleus. NINDS, 3 years, funded Dec. 1, $320,000 1988
- Emory-Georgia Tech Bioengineering Res. Ctr. With Drs. Bakay and Barrow, Dept. Neurosurgery, on recording from monkeys using the cone electrode. $20,000 1988
- Ionic Atlanta Inc, collaboration on project titled: Highly Adherent Ir and IrO2 Coatings on Neural Electrodes. $9,898 1988
- Emory-Georgia Tech Bioengineering Res. Ctr. With Dr. Nichols, Dept. Physiology, on developing a Rat Model of Plegia.
$27,000 1987
- Georgia Tech Research Corporation Capital Grant
$88,900 1986
- Center for Rehabilitation Technology $6,000 1986
- Electronics and Computer Systems Lab $10,000 1986
Administration
CEO Neural Signals Inc. Multiple collaborators. Multiple students.
Administrating Clinic in past. Now administering laboratory.
Chairman of the Animal Welfare Committee in Georgia Tech, 1987 to 1993.
Teaching:
Discussion group at Georgia Tech’s Applied Physiology dept. Nov 2012
Guest lecturer for Biomedical Instrumentation course organized by Dr. Paul Benkaser and Noberta Ezquirra in the Dept. of Electrical Engineering, GA Tech. Steve Potter 2009. Previous experience with team teaching and seminars for medical students.
Teaching Georgia State, guest lecturer, biomedical course director Prof. Brendan Allison.
Summary of Collaborations:
Present Active Collaborators:
Georgia Tech Research Institute, Dinal Andreasen, Cobb Co., Atlanta, Georgia.
Past Collaborators:
Georgia Institute of Technology. Prof. Mark Clements and Mr. Mark McCurry, Dept. of Electrical and Computer Engineering, Atlanta, Georgia.
Georgia Tech Research Institute, Brent Wagner and associates at GTRI, Atlanta, Georgia.
Emory University – Dr. Hui Mao, Department of Radiology, Atlanta, Georgia.
Gwinnett Medical Center: Dr. Michael Stetchison, and Dr. Princewill Ehirim MD, Neurosurgeon.
Rush University Hospital, Chicago, Dr. Roy Bakay (deceased: in past).
Dr. Jonathan Brumberg, Kansas State University, Kansas.
Prof. Prof. Frank Guenther and Mr. Jonathan Brumberg, Dept of Cognitive and Neural Sciences, Boston Uiversity, Boston, Massachusetts.
Prof. Earl Miller PhD and Scott Brincat, Cognitive Science Dept, MIT, Boston, Massachusetts.
Prof. Lee Miller and Mr. Jim Rebesco, Dept. of Physiology, Northwestern University, Chicago, Illinois.
Prof. Andy Schwartz and Dr. Meel Velliste, Dept of Physiology, University of Pittsburgh, Pittsburgh, Pennsylvania.
Dr. Crystal Gordon, North Carolina State University, Electrical Engineering, Raleigh, North Carolina.
Al Mann Foundation: Joe Schulman (Chief Eng) and David Hankin (CEO, Gen. Counsel)
Casey Stengel of Neuralynx Inc., Denver, CO.
Dr. Melody Moore, Georgia Tech and GA State Universities
Mr. John Goldwaithe, CRT, Georgia Tech.
Dr. Chris Russell, Dept. Neurology, Emory University.
Dr. Bakay, Neurosurgery, Emory University.
Dr. Steve Sharpe, GTRI, Georgia Tech.
Dr. Scott Crowgey, GTRI, Georgia Tech.
Dr. Don Humphrey, Physiology, Emory University.
Dr. Don Wigston, Physiology, Emory University.
Dr. T.R.Nichols, Physiology, Emory University.
Dr. Kent Davey, Georgia Tech and Veterans Administration
Dr. Sue Mirra, Neuropathology, Emory University.
Dr. Ed Schmidt, NIH, Bethesda, MD.
Jo Schulman, Director of Al Mann Foundation Research Labs.
Drs. Stoney and Doetsch, MCG, Augusta, GA.
Dr. Roger Nathan, Ben Gurion University, Israel.
Experience Summary
Prior to present positions:
1986 – present: Developed the Neurotrophic Electrode and performed six human implants, the last two being efforts to develop a speech prosthesis.
1993-97. Medical internship and Neurology Residency Program, Emory University. Completed June 30th. 1997.
1988-93 Yerkes Primate Center, Emory University.
1986-1993: Georgia Tech Biomedical Laboratory.
1983 to 1986. As a Research Associate with Prof. Donald Humphrey, I studied the function of the parvocellular division of the red nucleus using fiber sparing lesions in trained rats. Results suggest that the parvocellular red nucleus is important during compensation for rubrospinal tract lesions, while it does not play a role in movement control in the already compensated rat.
With Dr. Don Wigston I studied neural regeneration in the extensor muscles of axolotl hind limb. Current concepts hold that the target muscle is unimportant in guiding reinnervating fibers. Our data, however, shows the target is important, at least in the amphibian axolotl.
With Dr. Richard Nichols, I studied the role of brainstem regions (by stimulation of the red nucleus and inferior olivary nucleus), in the control of (1) background electromyographic activity and (2) the stiffness of the muscles acting around a joint, in the decerebrate cat preparation.
1980-83: Doctor of Philosophy in Physiology. Studied single cell activity of the magnocellular red nucleus in performing monkeys, and traced connections of this nucleus using HRP tracing techniques in monkeys.
1978-80: Post-doctoral Fellow. Investigated the role of the primate principal olivary nucleus in motor control, using a reversible lesion technique. I also developed a gas cooling system, plus an X-ray method of stereotaxic localization that is independent of the standard earbar zero reference point.
1976-78: Residency in training in Neurosurgery, Univ. of Western Ontario, London Ontario, Canada with Dr. Charles Drake.
1972-76: Residency training for boards in General Surgery (FRCSI), Ireland, various hospitals.
BIBLIOGRAPHY
REFEREED PUBLICATIONS
- Andreasen D, Gearin M, Kennedy N and Kennedy P. Assembly, impedance and recording characteristics of the NeuroNexus Neurotrophic Electrode (NXNE) with histological validation. Submitted to eNeuro 2024.
- Kennedy PR, Cervantes AJ. Recruitment and Differential Firing Patterns of Single Units During Conditioning to a Tone in a Mute Locked-In Human. Front. Hum. Neurosci., 21 September 2022, Volume 16 – 2022 | https://doi.org/10.3389/fnhum.2022.864983.
- Ganesh, A, Cervantes, A J and Kennedy PR, Classification and Fitting of words, silently spoken, as the basis of a speech prosthetic utilizing chronically recorded single units from the speech motor cortex. 2020.
- Gearin M and Kennedy PR. Histological confirmation of myelinated neural filaments within the tip of the Neurotrophic Electrode after a decade of neural recordings. Front. Hum. Neurosci. 21 April 2020 https://doi.org/10.3389/fnhum.2020.00111.
- Kennedy PR. To invade or not to invade, that is the question for brain computer interfacing. Current Trends in Neurology, 12:31-38, 2018.
- Kennedy P.R., Gambrell C, Ehirim P, and Cervantes A. Advances in the development of a speech prosthesis. Book chapter in Brain-Machine Interfaces: Uses and Developments accepted 2017.
- Kennedy P.R. Two tricks for longevity of human recording. Book chapter in Brain-Machine Interfaces: Uses and Developments accepted 2017.
- Kennedy P.R., 1 Dinal S. Andreasen,2 Jess Bartels,1 Princewill Ehirim,3 E. Joe Wright1, Steven Seibert1, Andre Joel Cervantes4 Validation of Neurotrophic Electrode long-term recordings in human cortex. Handbook of Biomedical Engineering. 2017.
- Kennedy P.R. Brain-machine interfaces as a challenge to the “moment of singularity”. Front Syst Neurosci. 2014 Dec 17;8:213.
- Sarmah E. and Kennedy P.R. Detecting Silent Vocalizations in a Locked-In Subject. Neuroscience Journal, Volume 2013 (2013), Article ID 594624.
- Kennedy, P.R., Andreasen D.S., Bartels, J., Ehirim P., Mao H., Velliste M.,Wichmann T.,Wright, E.J. Making the lifetime connection between brain and machine for restoring and enhancing function. Proceedings in Brain Research CH.1 August 2011.
- Kennedy, PR. Changes in emotional state modulate neuronal firing rates of human speech motor cortex: A case study in long-term recording. Neurocase 2011, 17(5), 381-393.
- Brumberg J., Wright EJ, Andersen D, Guenther FH and Kennedy PR. Classification of intended phoneme production from chronic intracortical microelectrode recordings in speech motor cortex. Frontiers in Neuroscience 5(65)1-14, 2011.
- Brumberg JS, Nieto-Castanon A, Kennedy PR, Guenther FH. Brain-Computer Interfaces for Speech Communication. Speech Commun. 2010 Apr 1;52(4):367-379.
- Guenther FH, Brumberg JS, Wright EJ, Nieto-Castanon A, Tourville JA, Panko M, Law R, Siebert SA, Bartels JL, Andreasen DS, Ehirim P, Mao H, Kennedy PR. A wireless brain-machine interface for real-time speech synthesis. PLoS One. 2009 Dec 9;4(12):e8218.
- Bartels J, Andreasen D, Ehirim P, Mao H, Seibert S, Wright EJ, Kennedy PR. Neurotrophic electrode: method of assembly and implantation into human motor speech cortex. J Neurosci Methods. 2008 Sep 30;174(2):168-76. Epub 2008 Jul 10.
- Using Human Extra-cortical Local Field Potentials to Control a Switch. Kennedy PR, Dinal Andreasen, Princewill Ehirim, Brandon King, Todd Kirby, Hui Mao, Melody Moore. J. Neural Eng. 1: 72-77, 2004.
- Correlations between human motor cortical local field potentials, action potentials, contralateral arm EMG activity and digit movements. Kennedy PR, Dinal Andeasen, Brandon King, Todd Kirby, Hui Mao, Melody Moore, Princewill Ehirim. J. Neural Engineering 2005.
- Computer Control Using Human Cortical Local Field Potentials. Kennedy PR, Kirby MT, Moore MM, King B & Mallory A. IEEE Trans on Neural Systems and Rehabilitation Eng. 12(3), 339-344, 2004.
- A Decision tree for Brain-Computer Interface Devices. Kennedy PR and Adams K. IEEE Trans on Neural Sys. & Rehab Eng. 11(2), 2003.
- Dynamic interplay of neural signals during the emergence of cursor related cursor in a human implanted with the Neurotrophic electrode. Kennedy PR and King B. CH 7 in Neural Prostheses for Restoration of Sensory and Motor Function. Eds. Chapin J and Moxon, K. CRC Press, 2001.
- Direct control of a computer from the human central nervous system. Kennedy PR, Bakay RAE, Adams K, Goldthwaite J, and M. Moore. IEEE Trans. Rehab. Eng., 8(2), 198-202, 2000.
- Restoration of neural output from a paralyzed patient using a direct brain connection. P.R.Kennedy and R.A.E.Bakay. NeuroReport 9,1707-11, 1998.
- Activity of single action potentials in monkey motor cortex during long-term task learning. Kennedy PR & Bakay RAE. Brain Research 760:251-4 (1997).
- Syphilitic gumma in an HIV positive patient. Roeske LC and Kennedy PR. New Eng J Med, 335(15):1123, (1996).
- Behavioral correlates of action potentials recorded chronically inside the Cone Electrode. P.R. Kennedy, R.A.E. Bakay and S.M. Sharpe. NeuroReport, 3:605-608, (1992).
- The Cone Electrode: Ultrastructural Studies Following Long-Term Recording. P.R. Kennedy, S.Mirra and R.A.E. Bakay. Neuroscience Letters, 142:89-94, (1992).
- Corticospinal, Rubrospinal and Rubro-olivary Projections: A unifying hypothesis. P.R. Kennedy, Trends in Neurosciences, 13(12):474-479 (1990).
- A long-term electrode that records from neurites grown onto its recording surface. P.R. Kennedy, J. Neuroscience Methods, 29 (1989) 181-193.
- Activity of primate Magnocellular Red Nucleus is related to hand and finger movements. Houk JC, Gibson AR, Harvey CF, Kennedy PR and Van Kan PLE. Behavioral Brain Research, 28 (1988) 201-206.
- The compensatory role of the parvocellular division of the red nucleus during reacquisition of coordinated motor tasks in operantly conditioned rats. Kennedy PR and Humphrey DR. Neuroscience Research (1987) 5:39-62.
- Parametric Relationships of Individual Digit Movements to Neuronal Discharges in Primate Magnocellular Red Nucleus. Kennedy PR. Brain Research (1987), 417:185-9.
- Light labeling of red nucleus neurons following an injection of peroxidase conjugated wheat germ agglutinin into the inferior olivary nucleus of the rat. Kennedy PR. Neuroscience Letters (1987), 74:262-268.
- Selective reinnervation of transplanted muscles by their original motoneurons in the axolotl. Kennedy PR and Wigston DJ. J. Neuroscience, (1987) 7(6):1857-1865.
- Anatomic and functional contrast between Magnocellular and Parvocellular Red Nucleus. Kennedy PR, Houk JC and Gibson AR. Brain Research (1986), 364:124-136.
- Eyeball retraction latency in the conscious rabbit measured with a new photodiode technique. Quinn KJ, Kennedy PR, Weiss C, and Disterhoft JF. J. Neuroscience Methods, (1984), 10:29-39.
- Movement programming depends on understanding of behavioral requirements. Brooks VB, Kennedy PR and Ross H-G. (1983) Physiol. & Behav. 31:561-563.
- Participation of the Principal Olivary Nucleus in Neocerebellar Motor Control. Kennedy PR, Ross H-G and Brooks VB. Expt. BR Res. (1982) 47:95-104.
- Chronic Implantation of Gas Cryoprobes in Monkey’s Brainstem. Kennedy PR and Ross H-G. J. Neuroscience Methods (1980) 2:411-418.
- X-ray Controlled Implantation of the Brain Stem. P.R. Kennedy, H.-G. Ross. J. Neuroscience Methods (1980) 2:411-418.
- The Rubro-Olivo-Cerebellar Teaching Circuit. PR Kennedy. Medical Hypotheses (1979) 5:799-807.
- The Olive and Central Control of Blood Pressure. PR Kennedy. Medical Hypotheses (1978) 4:593-595.
- Microvascular Neuronal Compression. PR Kennedy. Medical Hypotheses (1979) 5:275-6.
- New Data on Diastematomyelia. PR Kennedy. Journal of Neurosurgery (1979) 51 (3), 355-361.
Books and Book Chapters:
-
- Comparing Electrodes for use as Cortical Control Signals: Tiny Tines, Tiny Wires or Tiny Cones on Wires: Which is best? Kennedy PR. The Biomedical Engineering Handbook, Third edition. Ed.: Joe Brazino and Dan DiLorenzo, pp. 32-1 to 32.14, 2006. Update 2013.
-
- Dynamic interplay of neural signals during the emergence of cursor related cursor in a human implanted with the Neurotrophic electrode. Kennedy PR and King B. CH 7 in Neural Prostheses for Restoration of Sensory and Motor Function. Eds. Chapin J and Moxon, K. CRC Press, 2001, as above under refereed no. 24.
-
- Does cooling monkey’s inferior olive increase biceps stretch reflex? VB Brooks, PR Kennedy and H-G Ross. (1980), 361-6. In J. Courville, Lamarre and C deMontigny (Eds), The Inferior Olive. Raven Press, New York.
-
- Science Prediction book: 2051, published under a pseudonym Alpha O. Royal by thornislandpublishing 2012
-
- Children’s book: Get a Move on, Neuron! P.R. Kennedy, Published by Your Child’s Neuroscience Press, 1992. Republished by Choice Publishing Ireland as a cartoon book 2012.
-
- Teacher’s Manual: Get a Move on, Neuron! P.R. Kennedy, Published by Your Child’s Neuroscience Press, 1993.
-
- School Play: Get a Move on, Neuron! – The Play. P.R. Kennedy, Published by Your Child’s Neuroscience Press, 1993.
- Unlocking Erik: A freedom journey to restore speech to those with the locked-in syndrome. Published by Nova Science Publishers, April 2020.
Letters to the Editors:
Wish to die in end-stage ALS. Neurology, 2006 66(5):783-4.
Rubrospinal Tract. PR Kennedy. Neurology, 1997 48(5):1472-3.
Locked-in Patients. PR Kennedy, Neurology, 1994 44(2):366-7.
PRELIMINARY COMMUNICATIONS
- Histological confirmation of contents of Neurotrophic Electrode after recording for a decade. Marla Gearing & Kennedy PR, SFN Abstr. 2019.
- Classification and fitting of phonemes and words are the underlying basis of an invasive speech prosthetic that uses chronically recorded single units. Kennedy, PR, SFN Abstr. 2019.
- Using single unit bursts to decode audible and silent speech recorded chronically from a speaking human. P. R. Kennedy. SFN Abstr., 2018.
- Phil Kennedy, Tatiana Tom Tom1, Chad Gambrell1, Alex Kirillov2
Topography of human motor speech area using externally recorded 12-20 Hz beta peaks to identify articulatory movements and phoneme expression. SFN Abstr. 2017
- P. R. Kennedy, C. Gambrell and N. Shih, Detection of phonemes, short words and phrases from single units and 12-20 Hz frequency Beta band data during overt and covert speech recorded chronically from a speaking human. SFN Abstracts 2016.
- Phil Kennedy, Andre Cervantes. Modulation of single unit firing rates during phoneme production from the speech area of an intact human. SFN Abstracts 2015
- Phil Kennedy, Andre Joel Cervantes, Hui Mao. Simplified technique for localizing speech motor cortex implantation targets. SFN Abstracts 2014.
- Dan DiLorenzo, Phil Kennedy and Roy A.E.Bakay. Noninvasive EMG-Based Augmented Communication System For Aphasic Patients. SFN Abstracts 2013.
- Phil Kennedy, Roy Bakay, Andre Cervantes, Joe Wright, Jess Bartels, Steven Seibert, Dinal Andreasen, Princewill Ehirim, Mark Clements. Longevity of the Neurotrophic Electrode in humans. SFN Abstracts. 2013.
- T.H. Sanders, B. Matthews, M.A. Clements, P. R. Kennedy,
Discrimination between listening intervals and speaking attempts improves phoneme classification in a locked-in patient. SFN Abstrs. 2012.
- Elina Sarmah, Phil Kennedy, Detection of vocalization onset in a mute subject. SFN Abstr. 2012.
- 79. Phil Kennedy, Joe Wright, Jess Bartels, Steven Seibert, Dinal Andreasen, Roy Bakay, Princewill Ehirim, Brett Matthews, Mark Clements . Performance characteristics of the Neurotrophic Electrode in primates. Abstr. 2012.
- Kennedy P. The Speech Prosthesis: Status update. Neural Interfaces Conference, University of Utah, June 2012.
- Kennedy P. A Prosthesis for Restoration of Speech in Locked-In Patients. American Academy of Neurology, Annual Meeting, New Orleans 2012.
- M. Panko, S. Brincat, J. Brumberg, A. Salazar-gomez, J. Roy, S. Overduin, P. Kennedy, E. K. Miller, F. Guenther. Signal stability in chronic invasive brain-machine interfaces. Soc. for Neurosci. Abstrs, 2011
- T. H. Sanders, T. Wichmann, M. A. Clements, P. R. Kennedy. Speech phoneme detection and recognition from chronically recorded human motor cortex neurons. Soc. for Neurosci. Abstrs, 2011
- Using cross correlation analysis of recorded units to detect phonemes in human speech cortex. Phil Kennedy, Neural Signals Inc, Duluth, GA, Thomas Wichmann, Emory Univ. Dept Neurology, Atlanta GA, Joe Wright, Neural Signals Inc, Duluth, GA. SFN 2010.
- Modular Software Architecture for Neural Prosthetic Control. Velliste, M 1., Brumberg J2 and Kennedy P1 Neural Signals Inc., Duluth, GA 3Department of Cognitive and Neural Systems, Boston University, Boston, MA SFN 2009
- Human speech cortex [1]: Stability, variability, emotionality and multimodality of units recorded via the Neurotrophic Electrode. PR Kennedy1 D Andreasen1,2 J Brumberg3 J Bartels 1, D Felice1, EJ Wright1 . 1 Neural Signals Inc., Duluth, GA; 2 Georgia Tech, Atlanta, GA; 3 Boston Univ. MA SFN 2009.
- Human speech cortex [2]: Tuning of single units during listening and imagined singing of tones and musical notes using feedback. P. Kennedy1, D. Andeasen1,2, J. Brumberg1,3a, M. Clements2, F. Guenther3, J. Kim2, B. Mathews2, C. Ramos1, M. Velliste1,4, *E. J. Wright1. 1Neural Signals, Inc, Atlanta, GA; 2Georgia Tech., Atlanta, GA; 3Boston Univ., Boston, MA; 3Univ. of Pittsburgh, Pittsburgh, PA SFN 2009
- Advances in the development of the Neurotrophic Electrode. Siebert SA, Bartels J, Shire D, Kennedy PR, Andreasen A. SFN Abstr. 2008
- Novel Method for obtaining electric bio-signals for computer interfacing. Wright EJ, Siebert S, Kennedy PR, Bartels J. SFN Abst. 862.5, 2008
- Automatic detection of speech activity from neural signals in Broca’s area. Matthews, BA, Clements MA, Kennedy PR, Bartels J, Wright EJ, Andreasen A. SFN Abst. 2008
- Human Speech Cortex Recordings [6]: IC Development of Implantable Electronics D.S.Andreasen1,2, P. Hasler2,, C.Gordon3, J. Bartels1, P.R.Kennedy1, S.Siebert1, E.J.Wright1, SFN Abstracts 2007.
- Human speech cortex long-term recordings [5]: Formant frequency analyses J. S. Brumberg1, D. S. Andreasen2, J. L. Bartels2, F. H. Guenther1, P. R. Kennedy2, S. A. Siebert2, A. B. Schwartz3, M. Velliste3, E. J. Wright2 SFN Abstracts 2007.
65. Human Cortex Long-term Recordings [4]: Bayesian Analyses L.Miller4.J.Wright1, D.S.Andreasen1, J.L.Bartels1, J. Brumberg3, F.R.Guenther3, P.R.Kennedy1, J. Rebesco4, SFN Abstracts 2007. - Human Cortex Long-term Recordings [3]: Neural Net Analyses E.J.Wright1, D.S.Andreasen1,2, J.L.Bartels1, J. Brumberg3, F.R.Guenther3, P.R.Kennedy1, L.Miller4, J. Rebesco4, A.B.Schwartz5, S.A.Siebert1, M.Velliste5. SFN Abstracts 2007.
- Human Speech Cortex Recordings [2]: Inputs, Outputs, and Signal Stability J. L. Bartels1, D. S. Andreasen1,2, *P. R. Kennedy1, S. A. Siebert1, E. J. Wright1; 1: Neural Signals Inc., Duluth, GA 30096; 2: Georgia Institute of Technology, Atlanta, GA 30322. SFN Abstracts 2007.
- Human speech cortex long-term recordings [1]: spike sorting and noise reduction S.A. Siebert1, D.S.Andreasen2, J. Bartels1, J.Brumberg3,, F.H.Guenther3, P.R.Kennedy1, E.J.Wright1, SFN Abstracts 2007.
- Detecting patterns of neural signals from Broca’s area to produce speech in a locked-in subject. P.R.Kennedy, D.Andreasen,S.Seibert, E.J.Wright. SFN 2006.
- Towards conversational speech restoration in a locked-in patient by recording from Broca’s area with the Neurotrophic Electrode. P.R.Kennedy1., D. Andreasen1,2., E.J.Wright1., H. Mao3.,. P.Ehirim 4. SfN 2005.
59.Speech Prosthesis: Initial recordings from Broca’s area with the Neurotrophic Electrode in a locked-in patient. P.R.Kennedy, D.Andreasen,S.Seibert, E.J.Wright, H. Mao,. P.Ehirim. NIW, NIH, September 2005
58.Wright EJ, Kennedy PR. BCI Control for Locked-in Patients in Real World Environments. Presented at the BCI Conference, Rensellaerville NY 2005.
- Accurate Localization of Implant Targets in the Cerebral Cortices of Locked-in Subjects undergoing BCI Applications. P.R.Kennedy, Hui Mao, SFN 2004
- Different potential roles of Fast Transients and Local Field Potentials recorded through the Neurotrophic Electrode in humans. P.R. Kennedy, Dinal Andreasen, Neural Prostheses Workshop, submitted, 2003.
- Different potential roles of Fast Transients and Local Field Potentials recorded through the Neurotrophic Electrode in humans. P.R. Kennedy, Dinal Andreasen, Soc. Neurosci. Abstr. 2003.
- A comparison of Fast Transients and Local Field Potentials recorded through the Neurotrophic Electrode. P.R. Kennedy, Dinal Andreasen, Neural Control of Movement meeting, 2003.
- Directionality may be inherent in the Local Field Potentials (LFPs) recorded via the Neurotrophic Electrode in human cortex. P.R. Kennedy, B. King; M.T. Kirby; K. Adams. Soc. Neurosci. Abstr. 2002.
- Brain-Machine Interfaces: Can they teach us something? S. Mussa-Ivaldi, N.Hatsopoulos, P.R.Kennedy, M.Nicholelis, A.Schwartz and J.Wolpaw. Neural Control of Movement Meeting, Naples, FL, April 2002.
- Adams, KD, Goldthwaite, J, Plummer, T, Moore, MM and Kennedy, PR, (2001). Computer Control Using Surface EMG Signals”, RESNA Proceedings, Reno, NV, pp. 80-82.
- Motor Cortical control of a cyber digit by patient implanted with the Neurotrophic Electrode. P.R.Kennedy, B.King, M.T.Kirby, M.Blankowski and M.M.Moore*. Soc. for Neuroscience Abstr., 2001.
- The role of tactile feedback in the control of cortical neural signals two years after implantation in patient TT with mitochondrial myopathy. P.R .Kennedy, T .Kirby, K. Adams, B. King and A. Mallory. Neural Prostheses Workshop, NINDS, NIH, Oct. 2001.
- Directionality coding in human cortical area 4: Role of phase relationships of individual action potentials. P.R.Kennedy King B, Moore MM SFN Abstracts 2000.
- A Surface EMG Connection for Cursor Control and Morse Code. Adams, KD, Goldthwaite, J, Moore, MM and Kennedy, PR, (2000). RESNA Proceedings, Orlando, FL, pp 101-103.
- A research agenda for The Neural Signals brain-computer interfaces. MMMoore and P.R.Kennedy, ASSETS Conference, Washington, DC November 2000.
- Human factors issues in the neural signals direct brain-computer interface. M.M.Moore and P.R.Kennedy, 2002.
- Direct control of a computer from the human central nervous system. Kennedy P.R., Adams K, Bakay RAE, Goldthwaite J, Montgomery G and Moore M. BCI Conference New York, June 16th to 20th 1999.
- A direct brain connection for computer control. K. Adams, J. Goldthwaite, P.R. Kennedy, RESNA-99, June 25-29 1999.
- Neural Activity during acquisition of cursor control in a locked-in patient. P.R.Kennedy, R.A.E.Bakay, M.Moore, K,Adams, G.Montgomery. Soc. Neurosci. Abstracts, 1999.
- Cognitive Engineering: Early attempts to control a computer by directly interfacing with the CNS of a locked-in patient. P.R.Kennedy, R.A.E.Bakay, C.Russell & G. Montgomery. Neural Prostheses Workshop, 1998.
- Cognitive Engineering: Continuing experiences with implantation of the Neurotrophic Electrode in Locked-in patients. P.R.Kennedy and R.A.E.Bakay. Soc. Neurosci. Abstr., 1998.
- Cognitive Engineering: Using the Neurotrophic Electrode to access neural signals in locked-in patients: Experiences with initial human implantation. P.R.Kennedy and R.A.E.Bakay. International Meeting on Regeneration, Asilomar, CA 1997.
- Cognitive Engineering: Using the Neurotrophic Electrode to access neural signals in locked-in patients: Experiences with initial human implantation. P.R.Kennedy and R.A.E.Bakay. Soc. Neurosci. Abstr., 24(1)193, 1997.
- Plasticity of motor cortex action potentials during task learning in monkeys. P.R.Kennedy and R.A.E.Bakay. Soc. Neurosci. Abstr., 21(1)28, 1995.
- Morphological changes in rat red nucleus following compensation for limb lesions. P.R.Kennedy, N.Ishihara, L.Rutherford and J.Wilson. Soc. Neurosci. Abstr., 20(2)1436, 1994.
- The quietude of primate cerebral cortex is interrupted by microstimulation plus caffeine administration during chronic Cone Electrode recordings. P.R.Kennedy, L.L.Howell, R.A.E.Bakay, R.Verellan and J.Echard. Soc. Neurosci. Abstr., 19(1)777, 1993.
- “Get a Move on Neuron!” is now a teacher’s manual and a school play. P.R.Kennedy and T.L.Fountain. Soc. Neurosci. Abstr., 19(1)214, 1993.
- An implantable FM transmitter and amplifier powered by transcutaneous RF coupling for use in long-term prosthetic controllers. P.R.Kennedy, A.Hopper, C.Linker, R.Verellen, H.Yun and S.M.Sharpe. Neural Prosthesis Workshop Abstr., NIH, October, 1992.
- The Cone Electrode: Chronic Recording Techniques. P.R.Kennedy, A.Hopper, C.Linker, S.M.Sharpe and R.A.E.Bakay. Soc. Neurosci. Abstr., 18(1)217, 1992.
- Redefining Red nucleus: Anterograde projections from Red nucleus to the Inferior Olivary nucleus in rat. D.Yu and P.R.Kennedy, Soc. Neurosci. Abstr., 18(1) 311, 1992.
- A system for real time processing of neural signals for use as prosthetic controllers. P.R. Kennedy, A. Hopper, C. Linker and S.M. Sharpe. 14th. International Conference of the IEEE Engineering in Medicine and Biology Society meeting, Paris Oct. 29th. to Nov. 1st., 1992.
- A fun book for teaching neuroscience to 8-12 year old students. P.R.Kennedy, Soc. Neurosci. Abstr., 18(1)190, 1992.
- The Cone Electrode: Ultrastructural analysis of recorded tissue, behavioral correlates of neural activity, and development of a totally implantable system using transcutaneous power induction. P.R. Kennedy, A. Hopper, R.A.E. Bakay and S. Mirra. Poster presentation at the Neural Prosthesis Workshop, NIH, October 22-24, 1991.
- The Cone Electrode: Ultrastructural study following long-term recording. P.R. Kennedy, S. Mirra and R.A.E. Bakay. Soc. Neuroscience Abstr., 17(2):1018, 1991.
- Redefining rat Red nucleus: Multiple labelling of individual neurons from spinal cord, inferior olivary nucleus and cerebellar nuclei. D.Yu, S.Na, J. Wilson and P.R. Kennedy. Soc. Neuroscience Abstr., 17(1):469, 1991.
- Long-term recording of cortical units using the cone electrode in monkeys. Bakay R.A.E., Kennedy P.R. and Banks D.M. American Association of Neurological Surgeons Annual Meeting, 1991.
- Long-term recording of the same cortical units in monkeys using the cone electrode. Kennedy, P.R., Banks, D.M. and Bakay R.A.E. 21st Annual Neural Prosthesis Workshop, National Institutes of Health, October 1990.
- Long-term recording of cortical units using the cone electrode in monkeys. P.R.Kennedy, R.A.E.Bakay, N.Oyesiku and D.M.Banks. Soc. Neuroscience Abstracts, 16(2):1134, 1990.
- Re-defining rat red nucleus: Cytoarchitectural analysis of red nucleus neurons singly and doubly labelled from spinal cord and inferior olivary nucleus. C.L.Tucker and P.R.Kennedy. Soc. Neuroscience Abstracts, 16(1):729, 1990.
- Dynamic aspects of receptive fields of neurons chronically recorded in rat vibrissa cortex. D.Banks and P.R.Kennedy. Soc. Neurosci. Abstr. 15(1):962, 1989, and poster presentation at the Barrels Symposium, Phoenix Az, Oct.28-29 1989.
- Re-defining rat red nucleus: Cytoarchitecture and connectivity. C.L.Tucker, S.A.Lee, and P.R.Kennedy. Soc. Neurosci. Abstr. 15(1):405, 1989.
- The Cone Electrode: New concepts in long-term recording. Results in rat and monkey. P.R.Kennedy, 20th Annual Neural Prosthesis Workshop, NIH, Oct. 1989.
- Ion Assisted Ir and IrOxide Coating of Neural Electrodes. K.O.Legg, P.R.Kennedy and H. Solnick-Legg, 20th Annual Neural Prosthesis Workshop, NIH, Oct 1989.
- Robust Noise Suppression Techniques for Neural Signals. J.L.Lansford, P.R.Kennedy and J.E.Schroeder, IEEE Proceedings, 11(2/6) (1989) 681.
- The cone electrode: A Long-term Electrode that Records from Neurites. P.R. Kennedy. Society for Neuroscience Abstract, 14(2):1261, 1988.
- A new long-term recording electrode. P.R. Kennedy. Symposium: Spotlight on Research at Emory and Georgia Tech, Proceedings. April 11-13, 1988.
- Telemetry systems for high and low frequency biological signals. John Fanguy, Neal Hollenbeck, Philip Kennedy, Ann Patterson, Steve Sharpe. Symposium: Spotlight on Research at Emory and Georgia Tech, Proceedings. April 11-13, 1988.
- An Electrode that Records from Regenerated Neurites. Kennedy PR. International Symposium on Neural Regeneration. Asilomar, Ca. Dec. 6-l0, l987.
- Double Labeling of red nucleus neurons from dye injections into the inferior olivary nucleus and dorso-lateral funiculus of the spinal cord in rat. Kennedy PR (1987), Soc. Neurosci. Abstr., 13(2):852.
- The Rubro-Olivary projection in the rat. Kennedy PR (1986) Soc. Neurosci. Abstr., 12(1):353.
- Selective reinnervation of axolotl limb muscles by their original motoneurons. DJ Wigston and PR Kennedy (1986) Soc. Neurosci. Abstr. 12(1):541.
- Parvocelluar red nucleus is important during compensation for rubrospinal tract lesions in operantly conditioned rats. Kennedy PR, Newby D, Wallert M and Humphrey DR. (1985) Soc. Neurosci. Abstr., 2(2):1036, 1985.
- Significance of cortical input to the monkey red nucleus. Gibson AR, Harvey CF, Houk JC, Kennedy PR and Van Kan PLE. (1985) The Physiological Society and American Physiological Society joint meeting, Cambridge, England.
- Contrast between the 2 divisions and 3 cell types of monkey red nucleus. Kennedy PR, Gibson AR and Houk JC. (1983) Soc. Neurosci. Abstr. 10:(1), 537.
- Red nucleus activity related to finger movements. Kennedy PR, Gibson AR and Houk JC. (1983) Soc. Neurosci. Abstr., 9:(1), 224.
- Eyeball retraction latency in conscious rabbit measured with a new technique. KJ Quinn, PR Kennedy, C Weiss, and JF Disterhoft. (1983) Soc. Neurosci. Abstr., 9:(1), 642.
- Principal Olive (PO) cooling in behaving monkeys produces changes resembling those of Dentate dysfunction. Kennedy PR, Ross H-G and Brooks VB. (1981). Soc. Neurosci. Abstr. 7:640.
- Frequency changes of complex spikes of monkey’s cerebellar purkinje cells during cooling of the inferior olive. Ross H-G, Kennedy PR and Brooks VB. Can. Fed. Biol. Soc. Proc. (1979) 22:22.
- EMG and complex spike changes during cooling of the inferior olive. Brooks VB, Kennedy PR and Ross H-G. Soc. Neurosci. Abst. (1979), 5:98.
- Localization of brainstem targets in the Fascicularis Monkey by a histological X-ray matching method. Kennedy PR, Ross H-G and Brooks VB. Soc. Neurosci. Abst. (1978), 4:298.
Ph.D. Dissertation:
The control of finger movements: Structure, connectivity and function of the Monkey Magnocellular Red Nucleus. (1983). Northwestern University Graduate School of Medicine.
-
INTELLECTUAL PROPERTY
ISSUED:
-
- “Implantable Neural Electrode.” Patent #: 4,852,573, issued on August 1, 1989.
-
- “System and Method for Speech Generation from Brain Activity.” Serial number: 1/007,380. Filing date: 12/08/2003. Issued September 2007.
-
- Apparatus and method for detecting neural signals and using neural signals to drive external functions. Issued March 6, 2007. US: 7,187,967.B2
-
-
Medication Dispensing Device. Filed 5/29/2009. Issued August 30, 2011. US 8,009,040.B2
-
-
-
“Quantum Dot Neurotrophic Electrode Arrays”. Filed 3/6/ 2007. Serial Number 60/893,16. Foreign (PTC) filing: Neurotrophic Electrode Neural Interface Employing Quantum Dots.” Filing date: 3/5/2008. SSerial Number 12042742.
-
-
-
Neurotrophic Electrode System. Filed 12/15/2016. Serial number 15/380,097
-
-
- “Neural Electrode Array.” Serial #: 11/096,897. Filing date: 04/01/2005.
-
- “Software controlled electromyogram control system”. Serial #: 10/881,923. Filing date: 06/30/2004.
-
- “Detecting Neural Signals and using same to drive external functions.” Serial #: 10/675,703. Filing date: 9/30/2003.
- “Speech Prosthesis Employing Beta Peak Detection”; Serial number: 15/800,589 Filed November 2017.
PROPRIETARY KNOWLWDGE
-
Trophic factors used to grow neurites into the Neurotrophic Electrode tip.
INVITED PRESENTATIONS
2017 Panel discussion of future developments in AI and robots. Summit Knowledge conference. Dubai, UAE, Nov 22.
2016 Talk on Speech prosthesis seminar. Brown Univ., Rhode Island, USA
2013 Steps in the development of a speech prosthesis. Grand Rounds, Univ. of Oxford, England, Dept. of Neurology and Neurosurgery due Feb 8.
2013 Steps in the development of a speech prosthesis. Grand Rounds, Univ. of Oxford, England, Dept. of Neurology and Neurosurgery due Feb 8.
2013 Developing a speech prosthesis. Lecture, Univ. Newcastle, Dept. Neuroscience Feb 6
2013 Electrode design and implementation. Lecture, University College Cork, Ireland Feb 4
2012 Patent issues in the development of Speech Prosthesis, University College London.
2012 Development of Speech Prosthesis, Oxford Dept. Neurosurgery.
2012 Engineering the Speech Prosthesis, Tyndall National labs, Cork Ireland.
2012 Neural Prostheses Meeting, Univ. Utah. June 17th to 20th
2012 American Academy of Neurology, New Orleans, April 24
2011 Univ. of Utrecht, talk, May 20 to 21st, Utrecht The Netherlands.
2010 Building Better Brains: Neural Prosthetics and Beyond. Given Inst., Aspen. NYAS Sept
2010 Brain Machine Interfaces – Implications for science, clinic and society, Lund Univ. Aug.
2008 Invited seminar speaker Kansas University Medical Center, Sep 15.
2008 Invited Speaker: BrainGain symposium at Utrecht, the Netherlands, July 3-4.
2008 Distinguished Speaker: World Science Forum, Seoul, Korea, April 28-30
2008 Invited Speaker: Twelfth International Conference on Cognitive and Neural Systems, May 14-18
2007 Poster at Conference: Implanting Change: The Ethics of Neural Prosthetics, Penn State, August 26-28.
2006 Platform presentation at NIDCD Workshop on Brain Computer Interfaces for Speech Synthesis, May25-6.
2005 Platform presentation at Neural Interfacing Workshop, NIH, September
2004 Panel Speaker at Neural Prostheses Workshop, NIH, October.
2004 Seminar speaker at Case Western Res. Univ., Biomed. Eng. Dept. Sept.
2003 Panel Speaker at Neurotech Leaders Forum, San Francisco, CA, Sept.
2002 Panel speaker at RESNA Conference, Minneapolis, MN, June 28, 2002
2002 Panel speaker at BCI Conference, Rensellaerville, New York, June 2002
2002 Resource Forum Enterprise Award for Business Plans, Jan 31 2002. First place award.
- Wallace H. Coulter Prize presentation. Georgia Institute of Technology.
- 2000. Special Lecture on human control of computers. Arizona State University.
1999: BCI Conference. Invited speaker, Albany New York, June 16th to 20th.
- Merck US Human Health: “A Current Treatment Option in Migraine”, Young Harris, GA, May 4.
1999 State Univ. of NY, Potsdam University, February: Two talks on brain to computer interface.
1998: Dept. of Medicine, Morning report: Connecting to a paralyzed patient’s CNS. VA Hospital, 9/21/98.
1997: Society for Neuroscience, meeting on techniques in long term recording. November. New Orleans.
Grand Rounds: May 30th.: Experiences with initial human implantation of the Neurotrophic Electrode.
Grand Rounds Clinico-Pathological Conference:
Jan. 31st.: “Triple Header”: RMSF, Porphyria, AMIC. (Patel).
1996: Grand Rounds Short Case Presentations:
Sept. 20th: A case of neurocysticercosis. (Espiranzo)
May 10th: Devic’s Syndrome in an HIV patient (Whitten).
April 5th: Fatal Intracerebral Hemorrhage associated with cocaine use. (Gude)
March 8th.: Parietal (Optic) Ataxia in a patient with ICA stenosis and a discrete parietal stroke. (Grantling)
1995: Grand Rounds presentation on January 27th.: Emory Univ. Dept. of Neurology: Unlocking the Locked-in Patient with Direct CNS Access.
Grand Rounds short case presentations:
June 16th.: Intracranial hypotension due to root sleeve tear presenting as postural headache (Hiers).
May 26th.: ACA stroke possibly due to vasculitis (Shaw).
May 5th.: Cerebellar ataxia of unknown etiology (Rivers).
March 10th.: Neurosyphilis with VDRL negative CSF in an HIV positive patient (Wooten).
February 11th.: Neurosarcoid presenting with a dilated left lateral ventricle (Tommy Hall).
January 6th.: Severe anoxic encephalopathy with flat EEG secondary to EMD arrest (Hubbard).
1994: December 12th.: Seminar at Miami Project, Univ of Miami Florida: “Can chronic recording assist in connecting with the locked-in patient?”
Grand Rounds short case presentations:
October 29th.: [1] Bilateral Occipital lobe infarcts secondary to herniation due to hemorrhage in left frontal cortex. [2] Near-Gerstmann’s syndrome.
October 22nd.: [1] Unilateral brachaial and lumbar plexopathy in an uncontrolled diabetic, coumadinized with a PT of 90.
Parke-Davis Fellow at EEG-Based Brain-Computer Communication conference: 13th Annual Carmel Workshop on Cognitive Psychophysiology. Jan 1994.
1993: Chairman of Panel Discussion at NIH Neural Prostheses Workshop on Chronic Recording Electrodes. October 1993.
1992: “Can Chronic Neural Recordings be used to control Motor Prostheses?” Specialized Panel, Winter Brain Conference, Steamboat Springs, CO, February 1992.
1991: “A synthesis of cortical and subcortical mechanisms that cooperate during movements.” Frontiers in Neuroscience, October 18, 1991, Emory University, Deptartment of Anatomy and Cell Biology.
“Providing neural control signals to paralyzed persons.” Neuropathology Conference, Beaumont Hospital, Dublin, Ireland, January 4th., 1991.
1990: “Motor cortical signals as controllers of motor prostheses.” Medical Research Council Neurological Prostheses Unit, Institute of Psychiatry, De Crespigny Park, London SE5, 27th. July, 1990.
“Long term recording of single units from motor cortex of monkey for use as controllers of neural prosthetic devices.” Grands Rounds, Rehabilitation Institute, Emory University, Atlanta, May 25th.
“Long-term recordings for prosthetic applications.” Case Western Reserve University, Cleveland, Ohio. Applied Neural Control Research Meeting. May 21st. and 22nd.
“Accessing control signals from motor cortex for potential prosthetic use in humans.” Yerkes Research Center Seminar series. May 9th.
“The Cone Electrode: A long-term neural recording electrode with potential prosthetic applications.” Fourth Annual Convocation, Emory/Georgia Tech Biomedical Technology Research Center, April 11, 1990.
1989: “Towards human implantation of the cone electrode.” Bioengineering Center Seminar Series, Rm 303 CRB, Friday December 8th. 1989.
1988: Spotlight on Research at Emory and Georgia Tech.”Neural Signals for Activation of Paralyzed Muscles”. Georgia Institute of Technology, April 12, 1988.
2nd Annual Convocation of Emory/Georgia Tech Biomedical Technology Research Center: [1] “A system for Long-term Recording of Neural Signals”; [2] “Telemetry Systems for transmitting High and Low Frequency Biological Signals” (Delivered by John Fanguy). Emory University, April 13, 1988.
1987: Society for Neuroscience Atlanta Chapter.
“The Mammalian Red Nucleus: Current controversies.”
1985: Barrow Neurological Institute.
“Subcortical Control of Movement and Motor Learning.”
1983: Emory University Dept. of Physiology.
“Motor Control and Learning in the Parvocellular Red Nucleus.”